Abstract
Summary: Two patients with central pontine myelinolysis (CPM) were studied with diffusion-weighted MR imaging 1 week after onset of tetraplegia. In both patients, affected white matter showed hyperintensity on diffusion-weighted images associated with a decrease in apparent diffusion coefficient (ADC) values. In one patient studied serially, ADC values normalized by 3 weeks after tetraplegia. Early in the clinical course, diagnosis of CPM can sometimes be difficult. Hyperintensity on diffusion-weighted images may therefore have diagnostic utility. Decreased lesional ADC values support the notion that CPM is a consequence of relative intracellular hypotonicity.
Central pontine myelinolysis (CPM) is an uncommon consequence of certain metabolic derangements. These include rapid correction of hyponatremia, as well as hyperosmolar conditions, such as hyperglycemia. The microscopic appearance of CPM is loss of myelin with sparing of axons, without evidence of inflammation (1, 2). The abnormalities associated with CPM are readily identified on T2-weighted MR imaging scans (3). Little information is available regarding the characteristics of CPM revealed by diffusion-weighted (DW) imaging. Such information may be of diagnostic value and might also provide insight into the pathogenesis of this disorder. The current study considers these issues in reporting DW imaging results in two patients with CPM.
Case Reports
Case 1
A 27-year-old man presented to a community hospital with 2 days' delirium. He consumed alcohol daily for 1 year, including more than a case of beer per day for 3 months. Two days prior to admission, he developed nausea and vomiting and abruptly ceased alcohol intake. The admitting examination was remarkable for lethargy, confusion, and agitation. Serum sodium was 105 mEq/L. Cerebrospinal fluid and noncontrast head CT findings were unremarkable. Administration of intravenous saline increased serum sodium to 126 mEq/L over an 18-hour period. Over the next 3 days, he developed spastic tetraplegia and respiratory failure.
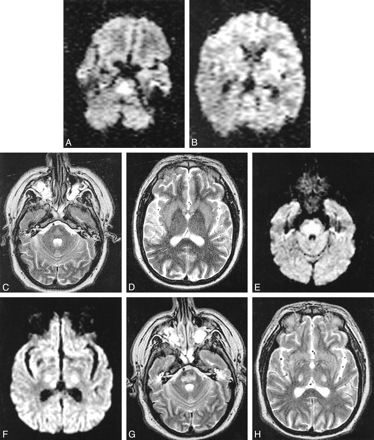
MR images obtained 6 days after developing tetraplegia showed mild T2 hyperintensity restricted to the pons, and DW images showed hyperintensity in the same region (Fig 1A–D). Apparent diffusion coefficient (ADC) maps were derived on a voxel-by-voxel basis using the following formula: [ln (T2 signal) − ln (DWI)]/1000. ADC was decreased in abnormal pontine regions (0.39 ± 0.14 × 10−3 mm2/s; mean ± SD) and was normal in unaffected white matter (0.90 ± 0.15 × 10−3 mm2/s). MR angiography of cervical and intracranial arteries was normal. The patient was transferred to University of Washington Medical Center and on day 21 after tetraplegia, repeat MR imaging (Fig 1E–H) showed areas of hyperintensity on T2-weighted images in the pons, as well as bilateral internal capsules. DW images also showed hyperintensity, but this was due to shine-through of T2 brightness because ADC was now normal in all of these areas. By 10 weeks, the patient's neurologic examination was entirely normal, apart from very mild abnormalities of tone and balance, plus a new intention tremor.
Case 1. MR images obtained using a 1.5-T magnet. Images for study 1 (A–D) were taken in an oblique axial plane 6 days after development of tetraplegia. Images for study 2 (E–H) were taken in an axial plane 21 days after development of tetraplegia.
A, DW image shows increased signal intensity in pons due to restricted water diffusion.
B, DW image is normal in the internal capsules bilaterally.
C and D, In the pons, there is little hyperintensity on the T2-weighted images and low ADC values, with mean ADC in basis pontis being 0.39 ± 0.14 × 10−3 mm2/s (mean ± SD) compared with 0.90 ± 0.15 × 10−3 mm2/s in unaffected white matter.
E, DW image shows increased signal intensity still present in the pons.
F, The DW image shows increased signal intensity present in the internal capsules bilaterally.
G and H, The DW abnormality is accounted for by shine-through from the hyperintense T2-weighted images. Mean ADC in basis pontis (1.09 ± 0.10 × 10−3 mm2/s) is normal compared with that of unaffected white matter (1.05 ± 0.09 × 10−3 mm2/s).
Case 2
A 60-year-old man with end-stage liver disease due to hepatitis B was admitted for hepatic encephalopathy. Five days prior to admission, the patient was ambulatory and had a serum sodium of 115 mEq/L. An initial neurologic examination showed confusion but normal motor function. From days 8–13 of admission, serum sodium ranged from 150 to 155 mEq/L. On approximately day 14, he developed a spastic tetraplegia. Head CT and Doppler studies of internal carotid and vertebral arteries were unremarkable.
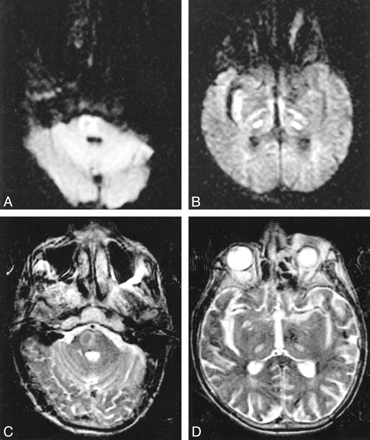
MR images obtained 7 days after developing tetraplegia (Fig 2) showed hyperintensity within the pons and the thalami bilaterally, consistent with CPM with extrapontine involvement. DW images also showed increased signal in these areas. ADC was decreased in the pons (0.62 ± 0.11 × 10−3 mm2/s) and in the thalamus (0.43 ± 0.09 × 10−3 mm2/s) compared with that of unaffected white matter (0.82 ± 0.12 × 10−3 mm2/s). The patient died 21 days after tetraplegia.
Case 2. Images obtained in the axial plane 7 days after development of tetraplegia. DW (A and B) and T2-weighted (C and D) images show increased signal intensity within the pons and the thalami bilaterally, consistent with CPM with extrapontine involvement. ADC is decreased in affected pontine (0.62 ± 0.11 × 10−3 mm2/s) and thalamic (0.43 ± 0.09 × 10−3 mm2/s) regions compared with that of unaffected white matter (0.82 ± 0.12 × 10−3 mm2/s). The patient died before repeat images could be obtained
Discussion
The clinical presentation of these patients is consistent with CPM (4) with extrapontine myelinolysis arising as a result of an increase in serum sodium. CPM was ultimately distinguished from basilar artery disease by the clinical history, the presence of spastic tetraplegia without tegmental signs, and the absence of cerebrovascular disease or significant ischemic insult. Furthermore, clinical recovery in patient 1 was excellent.
Early in the course, the clinical diagnosis of CPM can be challenging (4). Hyperintensity seen within the CPM lesions on DW images of both patients (Figs 1 and 2) after 1 week is of diagnostic utility. The DW findings do not distinguish CPM from ischemia (5), where ADC values are also decreased early in the course. However, ADC values within affected brain areas may be increased in other conditions that may clinically be confused with CPM. Conditions in which increased ADC values may be seen include certain brain tumors, acute disseminated encephalomyelitis, and multiple sclerosis (MS) (5–7). DW imaging is useful to distinguish these conditions from CPM because affected brain areas in patients with CPM show decreased ADC values (Figs 1 and 2). DW imaging is also useful to distinguish CPM from patients whose neurologic abnormalities arise from a psychiatric condition, in whom ADC values would presumably be normal. Both patients in the current report showed hyperintensity on T2-weighted images of the pons, but previous reports of patients with CPM have described a delay in appearance of T2-weighted MR imaging abnormalities (4, 8). DW images become sensitive at very early time points after ischemic brain injury (5). If the same is true for CPM, then diagnosis in patients with suspected acute CPM but normal T2-weighted MR images may be improved by the addition of DW imaging.
Experimental studies in animals have suggested a number of hypotheses regarding the pathogenesis of myelinolysis in CPM, including excessive brain dehydration (9), intramyelinic and neuronal edema (10), and cerebral vascular endothelial dysfunction (11). Clinical studies have suggested that some cases may be due to an overshoot of brain sodium during treatment of hyponatremia (12) or compression of myelin by swollen cellular elements (13). McKee et al (2) suggested that CPM arises when the extracellular fluid is made to be relatively hypertonic compared with intracellular fluid, regardless of the absolute osmolality or the identity of the dysequilibrated molecule. The mechanism of white matter dysfunction in CPM might also involve axonal swelling. Astrocytic swelling is also quite prominent in brain edema (14), and the contribution of glia to ADC values has not been precisely determined.
The current observations support the hypothesis that in humans, CPM is a consequence of a relatively hypotonic intracellular compartment. Intra-lesional ADC values were decreased in both patients 6–7 days after developing tetraplegia. There is experimental evidence that decreased ADC is associated with restricted water diffusion and increased intracellular water. For example, Anderson et al (15) found a decrease in ADC during osmotically driven axonal swelling, while cell shrinkage was associated with an increase in ADC. In MS, a condition with pathologic findings that partially overlap with CPM, ADC values within acutely demyelinated regions increase rather than decrease. Increased ADC values in MS may reflect expanded extracellular volume (7), whereas decreased ADC values in the current CPM patients likely reflect expansion of intracellular volume relative to extracellular volumes. The rise in ADC in patient 1 from day 6 to day 21 may reflect the rate with which certain intracellular osmolytes levels return to normal (12).
The differences in MR appearances between the pontine and capsular lesions in the first study of patient 1 (Fig 1A–D) might suggest that lesions at the two sites were of different ages, as has been described in patients with CPM (16); note that no osmolar insults occurred between the two MR studies. The cellular composition is quite different between pons and internal capsule. As a result, post-CPM histopathologic reactions (summarized by McKee et al [2]) may be dissimilar between these two areas and therefore may have contributed to these differences in MR appearance.
CPM is a disease that may arise in several settings and has a highly variable clinical outcome (4, 17). Early in the course, DW imaging may have diagnostic value. Results suggest relative intracellular hypotonicity, which has been hypothesized as a key event in the pathogenesis of CPM. The appearance of CPM lesions on T2-weighted MR images is often delayed (4, 8). Further studies are needed to determine the diagnostic utility of DW imaging immediately after onset of symptoms suggestive of CPM.
Footnotes
1 Address reprint requests to Steven C. Cramer, MD, University of Washington, Department of Neurology, 1959 N.E. Pacific Street, Box 356465, Seattle, WA 98195.
References
- Received December 18, 2000.
- Accepted after revision April 20, 2001.
- Copyright © American Society of Neuroradiology