Abstract
BACKGROUND AND PURPOSE: Retrograde leptomeningeal venous drainage (RLVD) in a dural arteriovenous fistula (DAVF) is associated with intracerebral hemorrhage, nonhemorrhagic neurologic deficit, or death, and recognizing the presence of this drainge is important. We investigated the MR findings of DAVFs draining into cerebellar cortical veins and compared these findings with those of conventional angiography.
METHODS: The MR and angiographic findings of six patients (five men, one woman; mean age, 73.4 years) with DAVF with RLVD into cerebellar cortical veins were reviewed retrospectively. Signal intensity characteristics, contrast material enhancement, topography of the lesion, and presence of signal voids were evaluated on MR images. Site of the shunt, feeding arteries, and draining veins were evaluated on angiograms.
RESULTS: In all patients, MR images showed high signal intensity on T2-weighted images and peripheral enhancement on gadolinium-enhanced T1-weighted images at the inferior aspect of the cerebellar hemisphere. A combination of posterior meningeal and occipital arteries was the most frequent blood supply (83%) for these DAVFs. In all six patients, the inferior hemispheric vein was the primary draining vein.
CONCLUSION: The characteristic MR findings of DAVF draining into cerebellar cortical veins represent venous congestive encephalopathy in the territory of the involved cortical vein.
Recognizing the presence of retrograde leptomeningeal venous drainage (RLVD) in a dural arteriovenous fistula (DAVF) is important because it is associated with an aggressive natural history that includes intracerebral hemorrhage, nonhemorrhagic neurologic deficit, or death (1–3). MR findings of surplus pial vessels associated with white matter edema may suggest venous congestive encephalopathy related to DVAF (4, 5). However, more than 50% of patients with DAVF with RLVD do not show MR evidence of white matter edema (4), and not all patients with white matter edema have angiographic evidence of venous congestion (5). In addition, the MR findings of venous congestive encephalopathy may be difficult to differentiate from venous infarction, demyelination, dysmyelination, or tumorous conditions (6). In our series, in five of six patients with DAVF with RLVD involving the cerebellar hemisphere, the initial diagnosis was possible tumor. The purpose of this study was to investigate the characteristic MR and angiographic findings of aggressive DAVFs involving the cerebellar hemisphere, with venous topographic analysis.
Methods
Two hundred thirty-seven patients with intracranial DAVF were evaluated by the University of Toronto neurovascular group since 1984. Among them, the MR images and angiograms of six patients (five men, one women; mean age, 73.4 years) who showed DAVFs with RLVD into cerebellar cortical veins were retrospectively reviewed by the authors. The MR findings that were analyzed included the topography of the lesion, signal intensity on T1- and T2-weighted images, gadolinium-based contrast material enhancement pattern, brain parenchymal hemorrhage, and presence of signal voids. Conventional angiography included selective bilateral vertebral, external carotid, and internal carotid arteries. The feeding arteries and venous drainage patterns were evaluated. Comparison of lesional MR topography and draining cortical vein territory was performed.
Results
All patients presented with a progressive cerebellar dysfunction, including incoordination of movements, ataxia, and/or loss of balance. One patient had a left-sided motor weakness. The right cerebellar hemisphere was involved in three patients and the left in three (Fig 1). Angiography was performed within 2 weeks of MR imaging, except for one patient in whom there was a 24-month interval. In all patients, high signal intensity on T2-weighted MR images was found in the inferior aspect of cerebellar hemisphere (Fig 2). On MR images, prominent tortuous signal voids over the cerebellar hemisphere were found in all patients. Three patients had vermian involvement, and one patient, who presented with left-sided weakness, had right thalamic high signal intensity on T2-weighted images. The central area of the lesion had low signal intensity on T1-weighted images in five of the six patients. In all patients, the periphery of the lesion was isointense on T1-weighted images and slightly hyperintense on T2-weighted images. On gadolinium-enhanced T1-weighted images, all patients showed diffuse peripheral enhancement (Fig 3). There was no evidence of cerebellar hemorrhage on the initial MR images. One patient developed massive cerebellar hemorrhage at follow-up and died.
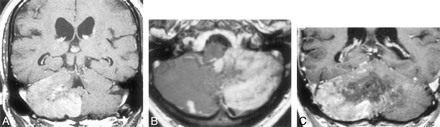
Patient 1, a 73-year-old man who presented with incoordination and ataxia.
A, Parasagittal T1-weighted MR image shows a low-signal-intensity lesion involving the posterior inferior aspect of the cerebellar hemisphere.
B, Axial T2-weighted MR image shows an ill-defined high-signal-intensity lesion (asterisk) in the left cerebellar hemisphere. Note multiple signal voids in the peripheral portion of the lesion.
C, Axial gadolinium-enhanced T1-weighted image demonstrates intense peripheral enhancement of the left hemispheric lesion, with a central nonenhancing area.
D, Left external carotid artery angiogram demonstrates DAVF (short thick black arrow) supplied by meningeal artery of cerebellar (Mening. a. of Cbll.) fossa coming from a neuromeningeal branch of the right ascending pharyngeal artery and left occipital artery (Occp. A.) draining through the inferior hemispheric vein (curved arrow).
A–C, Axial T2-weighted MR images in patient 5 (A), patient 2 (B), and patient 4 (C) show high signal intensity of the lesion, with various degrees of signal voids.
A–C, Gadolinium enhanced T1-weighted MR images in patient 3 (A, coronal), patient 2 (B, axial), and patient 4 (C, coronal) show diffuse peripheral enhancement.
On angiograms (Fig 4), four patients showed DAVF with direct RLVD to cerebellar cortical veins (inferior hemispheric vein or inferior vermian vein). Two patients showed tentorial sinus involvement that subsequently drained into cortical veins. Four patients showed RLVD directly into an inferior hemispheric vein, and two showed RLVD into an inferior vermian vein that then drained into the inferior hemispheric veins. Straight sinus thrombosis was demonstrated in one patient who had thalamic high signal intensity on T2-weighted MR images. The feeding arteries were multiple and included meningeal branches of the occipital artery (83%), artery of falx cerebelli (66%), meningeal artery of the cerebellar fossa (66%), and ascending pharyngeal artery (50%). One patient had supply from a C5 branch of the internal carotid artery. Clinical, MR, and angiographic findings are summarized in the Table.
A and B, Lateral (A) and anterioposterior (B) selective angiograms of the meningeal artery of the cerebellar (Mening. A. of Cbll.) fossa in patient 3 show a DAVF draining into the inferior vermian vein (Inf. Verm. V.) via a short tentorial sinus (Tent.S.).
C, Right vertebral angiogram in patient 4 demonstrates enlarged artery of the falx cerebelli (A.of Falx Cbll.) supplying a DAVF and draining into the right inferior hemispheric vein (Inf.Hemsp. V.) through the tentorial sinus (Tent.S.)
Clinical, MR, and angiographic findings of aggressive DAVF involving the cerebellar hemisphere
Discussion
Intracranial DAVFs are arteriovenous shunts located in the dura. Davies et al (1, 7) categorized them into benign and aggressive lesions based on the presence of RLVD. Benign DAVFs (no RLVD) often manifest with tinnitus or orbital venous congestion (chemosis, conjunctival injection), and most have an excellent natural history. On the contrary, about two-thirds of the aggressive lesions manifest with intracerebral hemorrhage or nonhemorrhagic neurologic deficit (1). The annual risk of intracerebral hemorrhage and nonhemorrhagic neurologic deficit after presentation is 8.1%/year and 6.9%/year, respectively (3). Therefore, angiographic evidence of RLVD is a definite indication for active treatment (1–3, 8–11), and the recognition of RLVD on MR images should prompt early angiography.
On MR images, dilated pial vessels (signal voids), diffuse white matter edema, and diffuse contrast material enhancement are signs of aggressive DAVFs (4). However, the MR findings may be variable according to the degree of venous congestion and the regional venous anatomy (collaterals). In addition, Willinsky et al (4) found that not all DAVF patients with angiographic evidence of venous congestion had high signal intensity on T2-weighted MR images.
Most patients with DAVF with RLVD have obvious abnormal signal voids on MR images. However, if there are no signal voids but brain edema and mass effect are evident in a cerebellar hemisphere, a number of diagnoses are entertained, including neoplasm and infarct. In our series, five of six cases were transferred to us with the provisional diagnosis of cerebellar tumor.
Four patients in our series showed RLVD directly into the inferior hemispheric vein. The other two patients showed RLVD into the inferior vermian vein, which eventually drained into the inferior hemispheric vein. Anatomic studies (12, 13) showed that the inferior hemispheric veins drain the inferior aspect of the cerebellar hemisphere and may terminate in the torcula, tentorial sinus, inferior vermian veins, or anterior hemispheric veins according to their locations. The inferior vermian veins also drain the inferior aspect of the cerebellar hemisphere and the vermis. They may drain into the straight sinus, transverse sinus, or torcula, either directly or through a short tentorial sinus. Thus, the inferior aspect of the cerebellar hemisphere and vermis are using either the inferior hemispheric vein or the vermian vein as their venous drainage route. Consequently, if these veins have venous thrombosis or high-pressure such as a DAVF with RLVD, the corresponding area would reveal the MR findings of venous congestive encephalopathy. All patient in our series had edema in the posteroinferior cerebellar hemisphere that exactly matched the territory of the inferior hemispheric vein.
The clinical symptoms and signs as well as MR findings of DAVF with RLVD can be reversible if treated successfully. In our series, three patients were treated successfully, two with open surgical disconnections and one with endovascular disconnection. The clinical symptoms of all three patients gradually improved after treatment. Follow-up imaging studies (one MR imaging and one CT) were available in two patients after successful treatment, and both showed the disappearance of brain edema.
The intracranial venous system is a complex three-dimensional structure and often asymmetric (14). Thus, determination of the involved cortical veins or dural sinus based on MR topography may not always be correct. Also, it is not easy to differentiate venous congestion due to aggressive DAVF from venous infarction due to focal cortical or dural sinus thrombosis based on the MR topographic analysis (15). Nonetheless, if the topography of the lesion on MR images corresponds to a particular regional venous territory, then the diagnosis of a DAVF with cortical venous reflux should be entertained. Furthermore, a surplus of pial vessels in addition to the appropriate topographic venous abormality supports the diagnosis of a DAVF.
Conclusion
Characteristic MR findings of aggressive DAVFs involving the cerebellar hemisphere included multiple signal voids, white matter edema, peripheral cortical contrast material enhancement, and a characteristic topographic involvement. Comparison of the MR and angiographic findings highlights the venous topography involved in venous congestive encephalopathy.
References
- Received November 20, 2002.
- Accepted after revision March 6, 2003.
- Copyright © American Society of Neuroradiology