Abstract
BACKGROUND AND PURPOSE: Gold has often been used in medicine because of its radiopacity and flexibility. To perform stent-supported coil embolization of intracranial aneurysms, we prepared a gold stent and examined its flexibility, radiopacity, and thrombogenic properties in comparison with a stainless steel device implanted in vitro and in vivo.
METHODS: Gold stents were prepared by plating gold on stainless steel stents as a template. Their mechanical properties and trackability in vitro were determined and compared with those of stainless steel stents of the same design. Twenty gold stents and two stainless steel stents were implanted in canine external carotid, vertebral, and renal arteries, as a muscle branch of the maxillary arteries, to examine their performance in vivo.
RESULTS: The gold stent exhibited much less radial force and greater flexibility than the stainless steel stent. It also demonstrated superior trackability and radiopacity in the experimental endovascular procedures in canines. Histologic examination showed good patency of the stented artery with slight endothelial hypertrophy.
CONCLUSION: Although there is still room for more radial strength, less influence on intimal hypertrophy, a more suitable flexibility, and a smoother surface, the superior trackability and radiopacity of gold stents seem to support use of this device for the endovascular treatment of intracranial aneurysms.
Intracranial wide-necked aneurysms represent therapeutic challenges in endovascular occlusion when electrolytically detachable coils are used, because the coil loops herniate through the broad neck into the parent artery. Endovascular approaches, such as the balloon-assisted coil-shape modeling method described by Moret et al (1) and three-dimensional, complex-geometry, electrolytically detachable coils (2), have been previously examined for managing wide-neck aneurysms. Other therapeutic approaches have been examined with the recent availability of a flexible intravascular stent developed for coronary artery use. Studies in which aneurysm models were used have also shown that the placement of the stent within the parent artery across the aneurysm orifice reduces flow vortices within the aneurysm and promotes intraaneurysm stasis and thrombosis (3–8). Stents are also expected to function as a scaffold to prevent coil protrusion into the parent artery lumen (8–11). These works demonstrated that the stent-supported treatment of aneurysms is a promising approach; however, stents specialized for intracranial endovascular treatment have not yet become commercially available. The properties required for an intracranial vascular stent are different from those for the stents developed for coronary artery use. Stents to be used for stent-supported coil embolization of intracranial aneurysms must have more flexibility and high radiopacity, but be different from coils, having lower thrombogenecity, a wide interstitial dimension, and so forth. High radial force is needed to treat atherosclerothic vascular lesions, but is not needed in stent-supported coil embolizations. The purpose of this research was to try the development of a stent specified for intracranial endovascular use and establishment of evaluation methods for an intracranial vascular stent.
In this study, a gold stent was developed for stent-supported coil embolization. Its mechanical properties and trackability in vitro were determined and compared with those of stainless steel stents of the same design. The performance of the gold stent in vivo was examined in canine external carotid, vertebral, and renal arteries instead of the swine coronary artery. The properties of the stent required for intracranial endovascular use were discussed in conjunction with the in vitro test method and in vivo animal model.
Methods
Preparation of the Gold Stent
The procedure used to prepare the gold stent is schematically shown in Figure 1. A stainless steel stent 3.0 mm in fully expanded diameter and 18.0 mm in length (ie, 3.0 × 18.0 mm) (Kaneka Corporation, Osaka, Japan) was used as a template for the preparation of a gold stent. It was electrically plated with copper in a current of 9.41 A/dm (2) for 30 minutes. The copper-plated stent was embedded in nylon elastomer resin and immersed into a gold-plating solution (Temperex 8400; Electroplating Engineers of Japan Ltd., Kanagawa, Japan) and gilded in a current of 0.78 A/dm (2)for 60 minutes. It was washed with distilled water and dried at 60°C. The synthetic resin was dissolved with hexafluoroisopropanol, and the copper-plating layer was removed with 2 N of nitric acid. It was washed with distilled water and baked at 400°C for 2 hours. Then, the gold plating to be used as a gold stent was removed from the stainless steel template. The gold stent was immersed in 2 N of nitric acid at room temperature for 2 hours and then extensively washed with distilled water. Finally, it was baked again at 400°C for 2 hours.
Schematic representation of the method used to prepare the gold stent.
Mechanical Test
The radial force of a stent should be sufficient to support the vessel’s internal form. In this study, the radial force of a stent is defined as the force generated when the stent is compressed to 70% of its fully expanded diameter. The flexibility of a stent is another important property for its use in tortuous vessels. A three-pointed bending test was employed to obtain an objective index of flexibility. A stent is more flexible as the force generated when a strain is bent 0.5 mm or smaller. The mechanical tests were done by using a compact table-top material tester (EZTest; Shimadzu Corporation, Kyoto, Japan). The above two tests were performed with the gold stents prepared, the stainless steel stent (used as a template for the gold stent in this experiment), Nir Elite (Boston Scientific, Natick, MA), Bx Velocity (Johnson and Johnson, Jacksonville, FL), S670 (Medtronic-AVE, 000, CA), and MultiLink Tristar (Guidant, Indianapolis, IN).
In Vitro Trackability Test
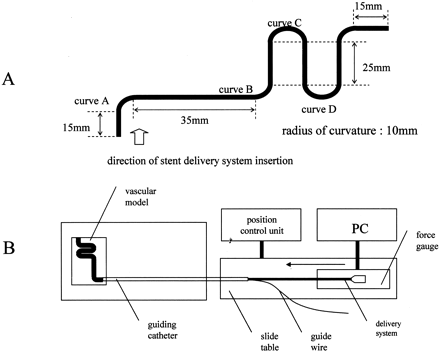
An in vitro vascular model for tortuous vessels was prepared by using a soft vinyl chloride tube 3.0 mm in diameter (Fig 2A). The trackability test system, also schematically shown in Figure 2B, was composed of a vascular model, a slide table for catheter insertion, a position control unit for the slide table movement, a digital force gauge (FGC-0.5; Nidec-Shimpo Corporation, Kyoto, Japan) for measurement of the catheter insertion force, and a personal computer for data collection. A 6F guiding catheter (Zuma II; Medtronic) was connected with the catheter insertion end of the vascular model placed in water heated to 37°C. A 0.014-inch guidewire (Balance; Guidant) was inserted into the guiding catheter and the vascular model. A stent delivery system was fixed to the digital force gauge set on the slide table. The slide table was moved toward the vascular model at a rate of 10 mm/s. The position of the tip of the stent delivery system and the force generated were recorded. The maximum attainment distance was 200 mm. The stent delivery system was able to go over curve D in Figure 2A.
A, In vitro vascular model for cerebral arteries.
B, Schematic illustration of the experimental system used for measuring trackability.
Stent Implantation and Poststenting Treatment
All animal experiments were conducted in accordance with policies set by the Animal Care and Use Committee of the Institute for Frontier Medical Sciences of Kyoto University. A total of four adult male hounds (7–9 months old; 17–18 kg in body weight) from Nosan:NRB (Yokohama, Japan) were used in the current investigation. Under general anesthesia (3 mg/kg body weight sodium pentobarbital via the venous route), orthogonal plane selective arteriography of the carotid, vertebral, and renal arteries was performed with a 6F guiding catheter (ENVOY; Johnson and Johnson Company, Tokyo, Japan) via the transfemoral route. Heparin (100 IU/kg body weight) was administered intravenously. Twenty gold stents and two stainless steel stents the same shape as those used as templates to prepare the gold stents were used in animal experiments. Each stent was mounted on a 2.6F (outer diameter)/2.0F (inner diameter), 14.0-atm maximum-inflation pressure coronary angioplasty balloon catheter (AeroCross-Fighter; Kaneka Corporation). The catheter, over a tapered 0.014-inch guidewire (205 cm in length, AGILITY; Johnson and Johnson Company, Tokyo), was advanced upward via the guiding catheter to the target site. The balloon was inflated to 4 atm for 30 seconds, deflated, and slowly withdrawn, leaving the stent in place. Before stent placement, the animals received 200 mg of ticlopidine daily for 4 days, and after stent placement, they received the same amount of ticlopidine daily for 2 weeks.
Angiographic Evaluation
Follow-up conventional angiography was performed 14 days after stent placement.
Histologic Evaluation
The stented arteries were resected for histopathologic study after the final angiographic study. Hematoxylin-eosin and elastica van Gieson staining were applied to identify fibrocellular proliferative tissue growth and to identify disruptions of elastic fibers after stent placement.
Results
Preparation of the Gold Stent
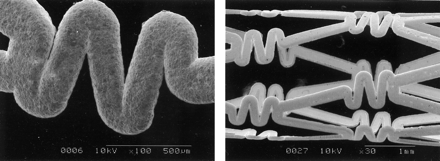
Electron microscopy showed that a gold stent with a nearly even thickness was uniformly produced (Fig 3). The thickness of the stent strut was measured to be 58 μm.
Scanning electron microscopy of the gold stent disclosed that a nearly even thickness was uniformly produced.
Mechanical Test
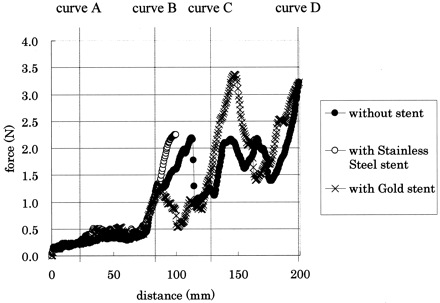
The radial force of the gold stent was measured at 10.3 mN/mm. The flexibility of the gold stent from the three-pointed bending test was gauged to be 0.28 N. These values for the other stents are shown in the Table. The values indicate that the gold stent demonstrated much less radial force and greater flexibility than the stainless steel stents.
Results of stent properties measurement
In Vitro Trackability Test
The stainless steel stent delivery system was able to pass through point A, but not point B. The flexibility of the stainless steel stent used to prepare the gold stent as a template was almost the same as that of other stents used clinically (Table), so it was strongly suggested that these stents (Nir Elite, Bx Velocity, S670, MultiLink Tristar) also would not demonstrate good trackability. On the other hand, the gold stent delivery system was able to go beyond point D. The force generated at the proximal end of the stent delivery system was slightly stronger than that of the delivery system alone (Fig 4). It was thought that the gold stent on the balloon catheter could be delivered through tortuous vessels. It was also suggested that the gold stent is suitable for intracranial endovascular use.
Results of trackability measurements. The gold stent demonstrated much less radial force and greater flexibility than the stainless steel stents.
Stent Implantation
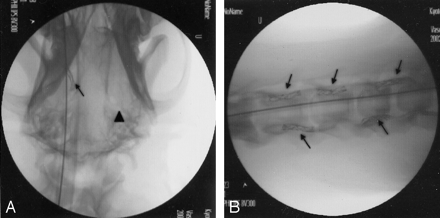
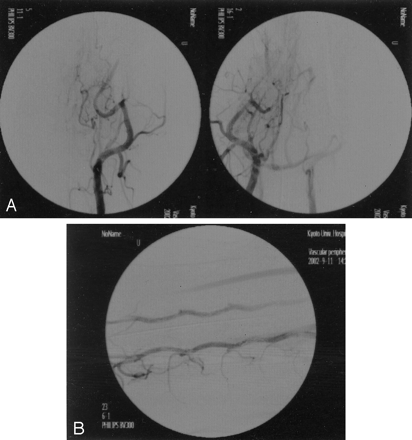
In total, 20 gold stents were placed in six external carotid arteries, 12 vertebral arteries, one renal artery, and one muscle branch derived from the left axillary artery, and two stainless steel stents were left in two external carotid arteries for comparison (Fig 5). At first, we were planning to place stents in the internal carotid artery, but this was very difficult because of its small diameter. We placed six stents in external carotid arteries. The external carotid artery was the most suitable artery for evaluation of the trackability of the stents. It appeared that the trackability of the gold stent examined in the external carotid artery was superior to that of the stainless steel stent, because the gold stent could be introduced into more distal portions. The radiopacity of the stainless steel stent was inferior to that of the gold stent (Fig 5A); however, follow-up angiography showed some deformation of stents that might be caused by the motion of the tongue. Other arteries, such as the vertebral artery and the renal artery, were used. Although the renal artery is almost free from external force, it was difficult to cannulate the catheter because of the sharp curve from the aorta to the renal artery. We found it difficult to cannulate the guiding catheter into the renal artery. When the gold stent was introduced into the renal artery, it became deformed because of the very sharp curvature from the aorta to the renal artery. The fragility of the gold stent might cause some difficulty when it is applied to sharply curved vessels. We, however, expect that the gold stent will be less affected going through the human carotid siphon, because the curvature of the human carotid siphon is not as tight as that from the aorta to the renal artery in the canine model. We found that vertebral arteries were the most suitable arteries for the gold stent examination. The gold stents did not become deformed when they were placed in the transverse processes (Fig 5B). This proved that the plasticity of the gold stents is not a problem for intracranial endovascular use.
Stent implantation.
A, Stent implantation (gold and stainless steel stents) in a canine model. A gold stent (arrow) was placed in the right distal external carotid artery, and a stainless steel stent (arrowhead) was placed in the left proximal external carotid artery. The radiopacity of the gold stent is superior to that of the stainless steel stent.
B, Stent implantation (gold stents) in canine bilateral vertebral arteries. Gold stents (arrows) were placed in a row in the bilateral vertebral arteries.
Angiographic Evaluation
Although some deformation of the gold and also stainless steel stents was observed at sites influenced by external force, follow-up conventional angiography demonstrated good patency at most stent placement sites (Fig 6).
Angiographic findings. Follow-up angiography performed 2 weeks after the stent placement shows good patency at each stented site (A, external carotid arteries; B, vertebral arteries). There is some deformation of both gold and stainless steel stents.
Histologic Evaluation
Twenty vessel segments treated with gold stents with approximately 5 mm of adjacent normal arterial tissue on each side were resected for histopathologic study after the final angiographic study. Histopathologic examination confirmed the angiographic findings ((Fig 7). The lumen of the stented artery was quite patent; however, mild endothelial hypertrophy was recognized around the strut of the gold stent.
Histologic findings of the vessel segments treated with gold stents. Histopathologic examination confirms the angiographic findings. The lumen of the stented artery is quite patent. Some endothelial hypertrophy is recognized around the struts of the stent (arrowhead). There are also artifacts in that the struts of the stent separate from the intima at the time of extraction (arrows).
Discussion
A new therapeutic approach to the endovascular treatment of intracranial aneurysms has been examined with the recent availability of flexible intravascular stents developed for coronary artery use (12–15). A stent functions as a scaffold to cover the orifice of aneurysms (8–11). Studies in aneurysm models have shown that the placement of the stent within the parent artery across the aneurysm orifice reduces flow vortices within the aneurysm and promotes intraaneurysm stasis and thrombosis (3–8). The reduction of flow velocity inside an aneurysm with large areas of stagnant flow depends on stent filament diameter and mesh density (7). Electrodetachable coils are preferentially used for the treatment of intracranial saccular aneurysms. It is difficult, however, to treat wide-necked intracranial saccular aneurysms by using only electrodetachable coils. Stent-supported coil embolization has been examined.
The tortuousness of the intracranical arteries is more complicated than that of the coronary artery, so one must develop more flexible stents with good trackability for intracranial use. A stent for the endovascular treatment of intracranial aneurysms must have good flexibility, minor thrombogenecity, a wide interstitial dimension, and a proper interstitial dimension to perform intraaneurysmal coil embolization between the stent struts and also to prevent herniation of the coil loops into the parent artery lumen. It also should have a different radiopacity from coils, because one can recognize the coil loops herniating through the broad neck into the parent artery between the struts of the stent.
Gold has often been used in medicine, such as for dental prostheses and in gold-plated implants. It has been also used in endovascular treatment to mark catheters, guidewires, balloons, and stents. In this experiment, we examined gold as a material with which to prepare a stent for treatment of intracranial aneurysms. We demonstrated the superior flexibility and radiopacity of the gold stent compared with a stainless steel stent. More distal portions of the external carotid artery could be accessed than with a stainless steel stent. We used vertebral arteries to evaluate the plasticity of the gold stents. The gold stents were not deformed when they were placed in the transverse processes, in which the stents in vertebral arteries were almost free from external force. This proved that the plasticity of the gold stents is not a problem for endovascular use. The gold stent was flexible enough to pass through the tortuous portion. Its increased flexibility and high visibility under radiography as compared with the stainless steel stent are at the expense of radial strength; however, a metal that demonstrates more elasticity than gold might be more suitable as material for a stent, because the gold stent was easily deformed by external force and could not be restored to its original shape because of its plasticity.
Most preclinical examinations of stents developed for the treatment of atherosclerotic coronary artery diseases have been performed in swine coronary arteries. In this study, we aimed to develop stents for stent-supported coil embolization of intracranial aneurysms. The properties required for our stent are quite different from those of the coronary stent as mentioned above. The swine coronary arteries seem not to be a suitable in vivo model to evaluate the function of the developed stent. The superior trackability and high radiopacity of the stents are important properties for the treatment of intracranial aneurysms. The in vivo tests were conducted to evaluate these two properties. The canine external carotid artery was a more suitable artery for evaluation of the trackability of the stents than the swine coronary artery.
Although the lumen of the stented artery was well patent, mild endothelial hypertrophy was recognized around the strut of the gold stent. The earliest clinical use of gold-plated stents was in the genitourinary tract (16). Initial studies have reported that the gold-coating may decrease thrombus formation as well as neointimal hyperplasia within the stents (11, 1, 18). Edelman et al (19) have demonstrated that the neointima that formed in gold-coated stents with thermal processing was thinner than that observed with stainless steel Palmaz-Schatz stents. Kastrati et al (20) have indicated that coating steel stents with gold had no significant influence on thrombotic events, however, gold-coated stents were associated with a considerable increase in the risk of restenosis. Several clinical studies (21–24) showed that a gold coating may increase the risk of restenosis after coronary stent placement. Rougher surfaces on stents induce thrombosis, mural injury, intimal hyperplasia (17), platelet adhesion (10), and altered endothelial cell migration (25). Heating gold-coated stents may remove adverse material-tissue interactions with possible interrelated effects of smoothing of the stent surface, removal of impurities, and strengthening of the gold-stent interface (19). In our experiment, a certain amount of intimal hypertrophy was recognized around some struts of the gold stents in the histopathologic examination. We suspect that this intimal hypertrophy is due to the mural injury induced by stent implantation or to the influence of the copper contaminating gold stents as an alloy in the process of plating. For the former, we should have used undersized stents instead of oversized ones. Most preclinical work for stents has been done in swine, but these stents are for the treatment of atherosclerotic coronary artery diseases. In this experiment, we have examined stents for stent-supported coil embolization. The stents should be placed at parent arteries of intracranial aneurysms. Stents that just fit the parent arteries seem to be best suited to the treatment of intracranial aneurysms with stent-supported coil embolization. Such stent placement is expected to cause neither mural injury nor intimal hyperplasia subsequently. The intimal hyperplasia that often accompanies the use of stents for the treatment of atherosclerotic artery disease may become only a minor problem. We used a canine model in this experiment. We expect the negative influence of copper to be removed by plating the surface of the stent with additional gold. In the next experiment, we will further refine the gold stent to influence less the intimal hypertrophy, improve flexibility, and create a smoother surface.
Conclusion
Gold was used to prepare a stent for the endovascular treatment of intracranial aneurysms. The increased flexibility and visibility of the gold stent as compared with the stainless steel stent were at the expense of radial strength. The gold stent demonstrates superior trackability and high radiopacity compared with a conventional stainless steel stent. Such properties make it suitable for passing through the human carotid siphon and provide a clear view in radiography.
Footnotes
This study was presented at the annual meeting of the Japanese Society for Intravascular Neurosurgery, Okinawa, Japan, December 4–6, 2002.
References
- Received January 31, 2003.
- Accepted after revision July 29, 2003.
- Copyright © American Society of Neuroradiology