Abstract
BACKGROUND AND PURPOSE: The relationship between brain β-amyloid and regional atrophy is still incompletely understood in elderly individuals at risk of dementia. Here, we studied the associations between brain β-amyloid load and regional GM and WM volumes in older adults who were clinically evaluated as being at increased risk of cognitive decline based on cardiovascular risk factors.
MATERIALS AND METHODS: Forty subjects (63–81 years of age) were recruited as part of a larger study, the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability. Neuroimaging consisted of PET using 11C Pittsburgh compound-B and T1-weighted 3D MR imaging for the measurement of brain β-amyloid and GM and WM volumes, respectively. All subjects underwent clinical, genetic, and neuropsychological evaluations for the assessment of cognitive function and the identification of cardiovascular risk factors.
RESULTS: Sixteen subjects were visually evaluated as showing cortical β-amyloid (positive for β-amyloid). In the voxel-by-voxel analyses, no significant differences were found in GM and WM volumes between the samples positive and negative for β-amyloid. However, in the sample positive for β-amyloid, increases in 11C Pittsburgh compound-B uptake were associated with reductions in GM volume in the left prefrontal (P = .02) and right temporal lobes (P = .04).
CONCLUSIONS: Our results show a significant association between increases in brain β-amyloid and reductions in regional GM volume in individuals at increased risk of cognitive decline. This evidence is consistent with a model in which increases in β-amyloid incite neurodegeneration in memory systems before cognitive impairment manifests.
ABBREVIATIONS:
- AD
- Alzheimer disease
- APOE
- Apolipoprotein E
- Aβ
- β-amyloid
- PIB
- Pittsburgh compound-B
- PIB−
- PIB negative
- PIB+
- PIB positive
Alzheimer disease (AD), the most common form of late-life dementia, is characterized by abnormal deposits of neurofibrillary tangles of τ protein and plaques of β-amyloid (Aβ) protein in the brain, eventually leading to neurodegeneration and cognitive decline. The accumulation of Aβ in the brain is believed to be a key factor in the development of AD, and recent evidence suggests that reduction of brain Aβ in the early stages of AD may slow down cognitive and functional decline.1 Therefore, there is a need to find biomarkers that identify individuals at risk of developing AD pathology who might benefit from therapeutic interventions before substantial irreversible neurodegeneration occurs.
Neuroimaging using PET and ligands specific for Aβ such as 11C-labeled-Pittsburgh compound-B (11C PIB) allows the measurement of brain fibrillary Aβ load in vivo. Previous studies have found increases in brain 11C PIB uptake not only in patients with AD but also in patients at risk of AD.2 The increases in 11C PIB uptake in these patient samples are significantly associated with brain atrophy.3,4 However, the evidence is mixed on the relationship of brain volumes and Aβ load in elderly subjects without clear cognitive impairment: Earlier studies have found both positive5,6 and negative associations7 or no associations.8 This inconsistency may be related to differences in methodology and the stage of AD pathology of the samples.
Here, we studied the relationships between brain Aβ and apolipoprotein E (APOE) ε4 carrier status with regional GM and WM volumes in a population-based sample of elderly individuals without manifest cognitive impairment but at high risk of developing dementia based on a cardiovascular risk factor profile. Earlier research suggests substantial regional variation in the accelerated brain atrophy related to early Aβ accumulation.9 Therefore, we hypothesized that increases in 11C PIB uptake are associated with specific patterns of brain volume loss. A better understanding of the relationship between Aβ and brain atrophy would not only elucidate AD mechanisms in at-risk subjects but also potentially help develop imaging-based identification of individuals who might benefit from early intervention.
Materials and Methods
Subjects
The subjects were recruited as part of the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (clinicaltrials.gov identifier NCT01041989). The study enrolled subjects 60–77 years of age with Cardiovascular Risk Factors, Aging and Dementia scores of at least 6 points10 and cognition at a mean level or slightly lower than that expected for age. At least 1 of the following criteria was required for inclusion: 1) Word List Memory Task of ≤19 words, 2) Word List Recall of ≤75%, or 3) Mini-Mental State Examination score of ≤26/30 points. In general, the subjects are representative of the Finnish elderly population with several risk factors for dementia.11,12 The exclusion criteria included major depression, dementia, or marked cognitive decline, Mini-Mental State Examination scores of <20, and symptomatic cardiovascular disease.
Here, we studied a subgroup of the above-mentioned sample (Turku University Hospital cohort), consisting of 40 subjects (21 men, 19 women; mean age, 71 ± 5.2 years). Before analyses, all neuroimaging data were evaluated for image quality. Written informed consent was obtained from all subjects who participated in the study. The study was approved by the Coordinating Ethics Committee of the Helsinki and Uusimaa Hospital District.
Clinical Measurements
The clinical measurements have been previously described in detail.13 The cognitive performance was evaluated using the modified Neuropsychological Test Battery,14 yielding a total composite z score and domain z score measures of memory, executive function, and processing speed. Total serum cholesterol and plasma glucose concentrations were determined enzymatically using commercial reagents and a clinical chemistry analyzer, Architect c8000 (Abbott Laboratories, Abbott Park, Illinois).
APOE Genotyping
Genomic DNA was extracted from venous blood samples with a chemagic Magnetic Separation Module I (Perkin Elmer, Waltham, Massachusetts) using magnetic beads. The APOE genotype was determined by polymerase chain reaction using TaqMan genotyping assays (Applied Biosystems, Foster City, California) for 2 single-nucleotide polymorphisms (rs429358 and rs7412) and an allelic discrimination method on the ABI 7500 platform (Applied Biosystems).15
Neuroimaging
11C PIB [N-methyl-11C-2-(4-methylaminophenyl)-6-hydroxybenzothiazole] was produced as described earlier.16 On average, 406 ± 110 MBq of 11C PIB was injected intravenously, and a scan from 60 to 90 minutes (3 × 10-minute frames) after injection was performed with an Ingenuity TF PET/MR scanner (Philips Healthcare, Best, the Netherlands). All images were reconstructed using a line-of-response row-action maximum likelihood algorithm with MR imaging–based attenuation correction using a segmentation-based algorithm with 3 tissue classes, including the head coil template used in the MR imaging protocol.17 The data were reconstructed using 2 iterations and 33 subsets. The image matrix size was 128 × 128 × 90, with an axial FOV of 256 × 256 mm and an isotropic voxel dimension of 2 mm. All quantitative corrections for PET data were applied, including scatter, randoms, attenuation, detector deadtime, and normalization. Neither time-of-flight information nor resolution modeling was applied in this study. Sagittal T1-weighted 3D MR imaging data were acquired for the measurement of brain GM and WM volumes, with TR = 25 ms, TE = 5.5 ms, and a reconstructed isotropic voxel dimension of 1 mm.
PET and MR Imaging Data Processing
The neuroimaging data were processed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12). The 11C PIB images were realigned and coregistered to the individual MR image and normalized to Montreal Neurological Institute space. 11C PIB uptake data were extracted using the standard automated segmentations by FreeSurfer 5.0 (http://surfer.nmr.mgh.harvard.edu).18 Regional 11C PIB uptake was quantified as a region-to-cerebellar cortex ratio during the 60- to 90-minute scan duration. The 11C PIB uptake values in the right and left hemispheres were averaged for data analysis.
MR imaging data processing for voxel-based morphometry analysis was performed using the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm.html), with default parameters for image processing. This included bias regularization and tissue classification and registration using linear (affine) and nonlinear transformations within a unified model.19 High-dimensional spatial normalization was accomplished using Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra. The analysis was performed on the volumes of GM and WM, multiplied by the nonlinear, but not linear, components derived from the normalization matrix. This procedure preserves actual local GM and WM volumes, accounting for individual brain size (modulated volume). The realigned and normalized GM and WM segments were smoothed with a Gaussian kernel with a full width at half maximum size of 8 mm.
Data Analysis
The PET images were visually interpreted by 2 experienced readers, and subjects were classified as either PIB positive (PIB+) or PIB negative (PIB−) on the basis of consensus agreement. The subjects with PIB+ findings had cortical 11C PIB retention in at least 1 region typically affected by β-amyloid deposition in AD, while the subjects with PIB− findings had only nonspecific 11C PIB retention in the WM. Brain GM and WM volumes were compared between the PIB+ and PIB− samples and APOE ε4 carriers and noncarriers voxel-by-voxel using an unpaired t test. The associations between 11C PIB uptake and brain GM and WM volumes, and interactions between 11C PIB uptake and APOE ε4 carrier status with regional GM and WM volumes were analyzed using whole-brain voxel-by-voxel multiple linear regression analysis. Age and sex were covaried in all analyses. Primary analyses were conducted using a composite 11C PIB uptake value, calculated as the average uptake in the following regions: anterior cingulate cortex, lateral temporal cortex, parietal cortex, posterior cingulate cortex, precuneus, and prefrontal cortex. Additionally, the 11C PIB uptake values for the precuneus, prefrontal cortex, and posterior cingulate cortex were used for regression analysis because they are among the first regions to show increases in 11C PIB uptake in mild cognitive impairment.2 The associations were determined separately in the PIB+ and PIB− samples.
Voxels with GM or WM values of < 0.1 were excluded from the analysis. A height threshold of P < .001 (uncorrected) was used across the whole brain for searching significant differences in brain GM and WM volumes (P < .05, family-wise error rate–corrected for multiple comparisons at the cluster level). Extent threshold was defined by the expected number of voxels per cluster based on random field theory. Cluster sizes were adjusted for nonstationary smoothness.20 In addition to the measures of GM and WM volume, microangiopathy-related hyperintensities in deep WM were evaluated on axial FLAIR images using a semiquantitative scale (0 = absence, 1 = punctate foci, 2 = beginning confluence of foci, 3 = large confluent areas).21
Statistical analyses were performed using SPSS, Version 23.0 (SPSS Statistics for Windows; IBM, Armonk, New York). Planned correlations were determined between clinical measures and GM and WM volumes in the PIB+ and PIB− samples. In these analyses, statistical significance was set at P < .05, and no correction for multiple tests was applied to these correlations.
Results
General Characteristics and APOE Genotype of the Subjects
The general characteristics of the subjects are shown in Table 1. APOE genotype was determined in 39 subjects. This sample consisted of 12 APOE ε4 carriers (ε2/ε4, n = 1; ε3/ε4, n = 10; ε4/ε4, n = 1) and 27 APOE ε4 noncarriers (ε2/ε3, n = 2; ε3/ε3, n = 25).
Sample demographics and clinical characteristicsa
Brain 11C PIB Uptake and GM and WM Volumes
The average global brain GM volume was 618 ± 73 mL in the PIB+ sample and 624 ± 52 mL in the PIB− sample, and the average brain WM volume was 502 ± 76 mL in the PIB+ sample and 520 ± 61 mL in the PIB− sample. There were no differences in the global GM and WM volumes between the samples (GM: F1,37 = 0.17, P = .68; WM: F1,37 = 1.21, P = .28). In the whole-brain voxel-by-voxel analyses, no significant differences were found in the GM and WM volumes between the PIB+ and PIB− samples, even when using a very lenient search threshold (P < .05, uncorrected).
In line with our earlier work, significant correlations were found between the regional 11C PIB uptake values and the composite 11C PIB uptake value in the PIB+ sample (all bivariate ROI correlations, P ≤ .001; Pearson r = 0.70–0.98). Therefore, the composite 11C PIB uptake value can be used as a proxy for overall brain Aβ load in this study.
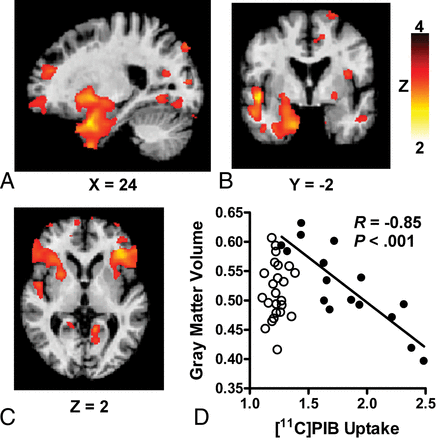
In the PIB+ sample, increases in composite 11C PIB uptake were associated with smaller GM volumes in the right temporal lobe (temporal pole, parahippocampal gyrus; peak Montreal Neurological Institute coordinates at [29, 14, −30], cluster size = 1170 mm3, z = 4.0, P = .04). At a lower search threshold (P < .01), this region also encompassed the right hippocampus and areas of the medial occipitotemporal gyrus (Fig A, -B). The increases in 11C PIB uptake in the precuneus were associated with smaller GM volumes in the left prefrontal lobe (inferior frontal gyrus; [−38, 32, 4], 262 mm3, z = 3.8, P = .02; Fig C). At trend level (P = .06), an association was found between increases in 11C PIB uptake in the prefrontal cortex and smaller GM volumes in the right temporal lobe (temporal pole, parahippocampal gyrus, amygdala; [29, 12, −30], 1390 mm3, z = 3.7). There were no regions with a significant positive correlation between 11C PIB uptake and GM volume. In the PIB− sample, no significant associations were found between brain 11C PIB uptake and GM volume (all regions, P > .05). No significant associations were found between 11C PIB uptake and regional WM volume in the PIB+ or PIB− samples. The main results are summarized in Table 2.
Summary of associations between 11C PIB uptake and brain regional GM volume
Associations between increases in 11C PIB uptake and reductions in GM volume in subjects with PIB+ findings at increased risk of cognitive decline. In subjects classified as positive for 11C PIB uptake, significant associations are found between increases in composite 11C PIB uptake and reductions in GM volume in the right temporal lobe (A and B) and increases in 11C PIB uptake in the precuneus and reductions in GM volume in the left prefrontal lobe (C). D, The significant negative correlation between composite 11C PIB uptake and GM volume in the right temporal lobe in the PIB+ sample (closed circles). No correlation is found in the PIB− sample (open circles). The R and P values were calculated using the average GM volumes extracted at P < .001.
APOE ε4 Carrier Status and GM and WM Volumes
No significant differences were found in global brain GM volumes between APOE ε4 carriers and noncarriers (F1,36 = 1.44, P = .24). The global WM volumes were significantly smaller in APOE ε4 carriers compared with noncarriers (F1,36 = 4.62, P = .04). In the whole-brain voxel-by-voxel analysis, no significant differences were found in regional GM volumes when comparing APOE ε4 carriers and noncarriers. Furthermore, no significant interactions were found between APOE ε4 carrier status and composite 11C PIB uptake with regional GM volume. Regarding regional WM, APOE ε4 carriers had significant reductions in WM volumes in the right parieto-occipital region compared with APOE ε4 noncarriers ([18, −87, 32], 4810 mm3, z = 4.7, P = .001). No significant regions were found in the opposite contrast (APOE ε4 carriers > APOE ε4 noncarriers). No interactions were found between APOE ε4 carrier status and composite 11C PIB uptake with regional WM volume.
Clinical Correlations
In the overall sample, larger global GM and WM volumes were associated with younger age (GM: r = −0.35, P = .01; WM: r = −0.37, P = .009). Larger global WM volumes were also associated with higher Mini-Mental State Examination scores at trend level (r = 0.28, P = .08). Correlations between regional GM or WM volumes and clinical measures, including WM hyperintensities, were short of significance (all P values > .05).
Discussion
PET imaging using ligands specific for Aβ, such as 11C PIB, allows the evaluation of AD pathology even before clinical symptoms emerge. However, a number of patients with PET scans positive for 11C PIB do not develop AD, indicating that other biomarkers are needed to accurately identify individuals who might benefit from pharmaceutical or life-style interventions.1,12 Earlier studies in patients with AD and its prodromal states have found significant correlations between 11C PIB uptake and brain volume loss,3,4,22,23 which is more closely related to the cognitive symptoms than Aβ load.6,8 Significant correlations have been described between brain Aβ accumulation and atrophy even in elderly subjects with no cognitive symptoms5,6; in fact, it has been suggested that this correlation is particularly strong at early stages of AD pathology.24,25 Our present data extend the previous observations by showing significant associations between increases in brain Aβ and GM loss in elderly subjects at high risk of cognitive impairment. However, some studies in cognitively healthy subjects have failed to find associations between 11C PIB uptake and atrophy8 or have even suggested a positive correlation between 11C PIB uptake and GM volume.7 Considering the distinct time scales of brain Aβ accumulation and GM loss, these discrepancies may relate to differences in the stage of AD pathology among the samples.
In the PIB+ sample, a significant association was found between increases in the composite measure of brain 11C PIB uptake and smaller GM volumes in the right temporal lobe, encompassing structures of the medial temporal lobe memory system. This finding is in line with previous work in cognitively healthy elderly subjects5,6,24,25 and suggests that asymptomatic elderly individuals who are at risk of cognitive decline and have a substantial brain Aβ load show signs of impending neurodegeneration in the temporal lobe. Although no significant associations were found between temporal lobe volume and cognition (Mini-Mental State Examination, total z score, and subscores of the modified Neuropsychological Test Battery), the significance of coexisting brain Aβ load and temporal lobe atrophy in clinically healthy elderly is highlighted by data suggesting that the adverse effect of these variables on cognition is synergistic.26 Therefore, it is likely that the subjects with PIB+ findings with temporal lobe atrophy in this study are at high risk of future cognitive decline.
In addition, increases in 11C PIB uptake in the precuneus in the PIB+ sample were significantly associated with GM volume reductions in the left prefrontal lobe. While studies on brain atrophy in the context of AD pathology have generally focused on medial temporal lobe structures, AD has also been shown to be associated with atrophy in a number of other brain regions, including the frontal lobes, precuneus, and posterior cingulate cortex.27,28 Furthermore, brain Aβ load in cognitively healthy elderly has been shown to be associated with GM volume loss in the frontal, parietal, and temporal lobes.5,27 There is even evidence suggesting that emerging Aβ pathology in cognitively healthy elderly is particularly associated with frontoparietal atrophy, while atrophy in the temporal lobe structures accelerates later as clinical symptoms begin to manifest.29 The mechanisms behind the regional differences in Aβ-associated atrophy are not well-understood: Potential mechanisms include differences in the afferent and efferent connections and vulnerability to Aβ-related toxicity.
Earlier studies have shown that the APOE ε4 allele is associated with CSF Aβ levels and changes in brain GM and WM in mild cognitive impairment and AD.30⇓–32 Although regional reductions in WM volumes were found in APOE ε4 carriers compared with noncarriers, the APOE ε4 carrier status had no effect on the relationship between 11C PIB uptake and GM volume, corroborating the results from previous studies conducted in clinically healthy elderly and subjects with mild cognitive impairment.33,34 These findings are in line with evidence showing that while APOE ε4 carrier status has a major effect on Aβ deposition, the effects on atrophy are subtle and are mediated by both Aβ-dependent and Aβ-independent mechanisms.35
Finally, this study has a few limitations. First, because the subjects were clinically selected to represent an elderly population with several risk factors for dementia, it is likely that they have mixed pathologies; conversely, some of the pathologies related to cognitive impairment may not have been considered in the current study. Second, the sample size was relatively small for the evaluation of associations between GM and WM volumes and clinical variables and the effects of APOE ε4 on the relationship between 11C PIB uptake and GM and WM volumes. Third, the cross-sectional data do not allow determining whether the subjects with PIB+ findings with impending temporal lobe atrophy develop cognitive impairment later on. Therefore, replication of these findings in larger samples as well as longitudinal studies are needed to determine the predictive power of 11C PIB PET and GM volume in cognitive impairment in at-risk elderly individuals.
Conclusions
Our results show that elderly individuals who are at increased risk of cognitive decline based on cardiovascular risk factors and have PET scans positive for 11C PIB exhibit reductions in regional GM volume in proportion to increases in brain Aβ load. Our findings are consistent with the model in which brain Aβ accumulation incites neurodegeneration before cognitive decline manifests. Furthermore, the results suggest that the brain Aβ-associated GM loss affects both the medial temporal lobe memory system and the neocortex. Together this evidence emphasizes the importance of finding biomarkers that identify individuals at risk of developing AD pathology who might still benefit from therapeutic interventions.
Acknowledgments
The assistance of the personnel of the Turku PET Centre in acquiring PET and MR imaging data is gratefully acknowledged.
Footnotes
Disclosures: Ilkka Martikainen—RELATED: Grant: Finnish Governmental Research Funding for Tampere University Hospital.* Nina Kemppainen—RELATED: Grant: Turku University Hospital, the Finnish Medical Foundation, the Sigrid Jusélius Foundation, the Maud Kuistila Foundation, the Paulo Foundation; UNRELATED: Employment: Turku University Hospital. Hilkka Soininen—RELATED: Grant: Academy of Finland*; UNRELATED: Board Membership: AC Immune; Consultancy: Merck. Tiia Ngandu—RELATED: Grant: Finnish Medical Foundation.* Miia Kivipelto—UNRELATED: Payment for Lectures Including Service on Speakers Bureaus: Nestlé. Juha O. Rinne—RELATED: Grant: Sigrid Jusélius Foundation, Comments: unrestricted academic grant*; UNRELATED: Consultancy: Clinical Research Services Turku Ltd, Comments: fee for serving as a consultant neurologist. *Money paid to the institution.
This study was supported by Finnish Governmental Research Funding for Turku University Hospital and Tampere University Hospital; the Finnish Medical Foundation; the Sigrid Jusélius Foundation; the Maud Kuistila Foundation; the Paulo Foundation; the Research Council for Health of the Academy of Finland (15762, 259615, 278457, 287490, 294061; and Responding to Public Health Challenges Research Program grants 129395, 129397, 129421, 129416, 129401); the La Carita Foundation; the Alzheimer's Association (grant HAT-10-173121); the Juho Vainio Foundation; the Novo Nordisk Foundation; the Finnish Social Insurance Institution; the Ministry of Education and Culture, Finland; the Swedish Research Council; the Alzheimer's Research and Prevention Foundation, United States; the AXA Research Fund; the Sheikha Salama bint Hamdan Al Nahyan Foundation; the Academy of Finland for Joint Program of Neurodegenerative Disorders–prevention (Multimodal preventive trials for Alzheimer's Disease); the Swedish Research Council; and the Swedish Research Council for Health, Working Life, and Welfare.
Indicates open access to non-subscribers at www.ajnr.org
References
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
- 8.
- 9.
- 10.
- 11.
- 12.
- 13.
- 14.
- 15.
- 16.
- 17.
- 18.
- 19.
- 20.
- 21.
- 22.
- 23.
- 24.
- 25.
- 26.
- 27.
- 28.
- 29.
- 30.
- 31.
- 32.
- 33.
- 34.
- 35.
- Received November 9, 2017.
- Accepted after revision October 12, 2018.
- © 2019 by American Journal of Neuroradiology