Abstract
Spinal CSF leak care has evolved during the past several years due to pivotal advances in its diagnosis and treatment. To the reader of the American Journal of Neuroradiology (AJNR), it has been impossible to miss the exponential increase in groundbreaking research on spinal CSF leaks and spontaneous intracranial hypotension (SIH). While many clinical specialties have contributed to these successes, the neuroradiologist has been instrumental in driving this transformation due to innovations in noninvasive imaging, novel myelographic techniques, and image-guided therapies. In this editorial, we will delve into the exciting advancements in spinal CSF leak diagnosis and treatment and celebrate the vital role of the neuroradiologist at the forefront of this revolution, with particular attention paid to CSF leak–related work published in the AJNR.
Spinal CSF leak care has evolved during the past several years due to pivotal advances in its diagnosis and treatment1. To the reader of the AJNR, it has been impossible to miss the exponential increase in groundbreaking research on spinal CSF leaks and spontaneous intracranial hypotension (SIH). While many clinical specialties have contributed to these successes, the neuroradiologist has been instrumental in driving this transformation due to innovations in noninvasive imaging, novel myelographic techniques, and image-guided therapies. In this editorial, we will delve into the exciting advancements in spinal CSF leak diagnosis and treatment and celebrate the vital role of the neuroradiologist at the forefront of this revolution, with particular attention paid to CSF leak–related work published in the AJNR.
Spinal CSF Leak Types
Classifying the different types of spinal CSF leaks has contributed to our better understanding of their anatomy and pathophysiology and has led to advances in diagnosis and treatment. There are 2 main types of spontaneous spinal CSF leaks encountered in clinical practice: dural tears (ventral and lateral/posterior) and CSF-venous fistulas (CVFs).2,3 Ruptured meningeal diverticula are an additional leak type that was discovered, but many diverticular leaks reported in the literature were likely lateral dural tears that mimicked a diverticular leak due to the arachnoid billowing through the dural defect.4,5
Spinal CSF Leak Publications
Spinal CSF leak research has been of recent interest in the neuroscience literature. To quantify this interest, we performed PubMed searches using the terms “spontaneous intracranial hypotension” or “CSF leak” or “CSF-venous fistulas” or “myelography” and “journal name.” The results were filtered between January 2020 and June 2024, and entries unrelated to spinal CSF leaks were excluded. Several neuroscience journals with known CSF leak publications were queried in the search, and any journal with ≥5 publications was included.
AJNR had the highest number of publications (n = 49), while Headache had the second highest with approximately one-third of the publications (n = 16) (Table). Of the 11 total journals included, there were 5 radiology journals. The 49 AJNR spinal CSF leak publications constituted approximately 3% of all 1647 AJNR publications during the 3.5-year time-frame. An analysis of spinal CSF leak publications in AJNR this century also shows a sharp increase in frequency during the past 5 years (Fig 1).
Number of spinal CSF leak publications in AJNR from 2000 to June 2024.
| Journal | Publications on CSF Leak, CVF, or Myelography Related to SIH between January 2020 and June 2024 |
|---|---|
| American Journal of Neuroradiology | 49 |
| Headache | 16 |
| Neurology | 13 |
| Clinical Neuroradiology | 12 |
| Interventional Neuroradiology | 12 |
| Neuroradiology | 10 |
| Journal of Neurointerventional Surgery | 9 |
| Cephalalgia | 7 |
| Neurology Clinical Practice | 7 |
| American Journal of Roentgenology | 6 |
| Journal of Neurosurgery Spine | 5 |
| Others | <5 |
Number of recent spinal CSF leak publications by medical journal
Not only are spinal CSF leak articles frequently published in the AJNR, but they are also commonly cited. On the AJNR homepage, under the “Most Cited” tab, 3 of the 5 articles listed are related to spinal CSF leaks,6⇓–8 and there are 5 additional articles listed in the full AJNR list at the time of writing.9⇓⇓⇓⇓–14
Why All the Interest in Spinal CSF Leaks?
One of the major reasons spinal CSF leak publications are topical is that they can be challenging to diagnose and treat. Physicians of many specialties have struggled with this entity: how to make the diagnosis, how to localize a spinal leak, what treatment to pursue, and what to do when no leak is observed. There are 3 main research breakthroughs that have ameliorated some of these challenges and have propelled interest in the field. First, improved myelographic techniques have resulted in more precise localization of CSF leaks due to dural tears, allowing more targeted and effective percutaneous and surgical treatments.
Second, the discovery of CVFs has resulted in a paradigm shift in the approach to a patient with suspected SIH. CVFs represent an abnormal connection between the spinal subarachnoid space and an epidural vein, which abnormally shunts CSF into the venous system without an associated spinal epidural fluid collection. CVFs were first reported in 2014,15 but it was not until it was realized that lateral decubitus myelography resulted in a several-fold increase in their detection that this diagnosis was made much more frequently in clinical practice.3,6,16,17 Because of our improved understanding and better detection of CVFs, many patients in whom the CSF leak source could not be verified were subsequently diagnosed with a CVF.18 Although once thought to be rare, studies have suggested that CVFs are possibly the most common type of CSF leak in patients with SIH.10 The pathogenesis of CVFs is still being studied, but potential associations have been observed between pre-existing intracranial hypertension as well as spinal degenerative disease.19,20 Lastly, innovations in CVF treatment, driven by neuroradiologists, further amplified excitement within the field of SIH. Surgery was the initial treatment modality described for CVFs, and while effective, is invasive and may require additional surgeries, as patients with CVFs may rarely develop same site recurrence or new CVFs after treatment. Subsequent work describing targeted fibrin glue occlusion and transvenous embolization were pioneered specifically for CVFs and resulted in substantial enthusiasm given their minimally invasive nature.
Advanced Myelography
Before the myelographic work-up, T2-weighted MR imaging of the spine is performed to evaluate the presence or absence of an extradural collection. This has largely replaced traditional myelography, whereby iodinated contrast is injected under fluoroscopy, allowed to diffuse evenly throughout subarachnoid space, and imaged after a substantial delay with the patient in the prone or supine position.21 While traditional myelography identifies the presence of an extradural fluid collection, it lacks the temporal resolution to precisely localize the site of the dural defect. Instead, once a CSF leak is suspected, dynamic or decubitus CT myelography (CTM) or digital subtraction myelography (DSM) currently represent the 2 main modalities used for spinal CSF leak detection. If an extradural collection is observed on the spine MRI, a dural tear is suspected and the patient is either positioned prone or in the decubitus Trendelenburg position, depending on the suspicion of leak type, typically using either a wedge or pillows. As an alternative, an innovative adjustable positioning device was invented to help achieve adequate angulation during myelography.22 After correct positioning of the patient, a lumbar puncture with contrast administration is performed and rapid scanning is performed to capture the transition of contrast from the subarachnoid to extradural spaces, which represent the CSF leak site (Figs 2 and 3). CTM for dural tears is sometimes called “ultrafast” or “dynamic,” given that several successive temporally progressive acquisitions are performed while simultaneously injecting contrast to identify the leak site. More recently, single-scan acquisitions with small contrast volumes have been described to achieve the same success with less radiation exposure.23
A ventral dural tear on a dynamic CT myelogram in a 34-year-old woman showing the direct outflow of the contrast medium (open arrow, A and B) within seconds into the ventral epidural space at the T3–T4 level. The contrast medium then flows cranially within the epidural space (solid arrows, A). The underlying cause is a calcified disc at the T3–T4 level (open arrowhead, A and B).
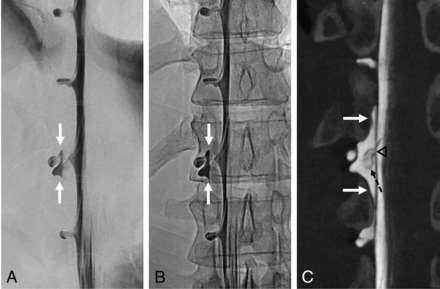
Digital DSM and conebeam CT of a lateral dural tear in a 36-year-old man. DSM with the patient in the right lateral decubitus position in an anterior-posterior projection suggests a small epidural contrast medium egress, which is unchanged in the subsequent single radiograph (white solid arrows, A and B). C, conebeam CT that follows a few minutes later confirms the epidural accumulation of contrast medium in the coronal view (white solid arrows) at the right T12–L1 level. In addition, a cyst-like structure can be seen within the contrast collection, corresponding to an arachnoid layer (dashed black arrow, C) herniating through a lateral dural tear in the axilla of the exiting nerve root sleeve (black arrowhead, C), which was later confirmed by surgery.
Decubitus myelography with CTM or DSM currently serve as the mainstay imaging modalities for CVF detection after its initial discovery with prone technique. A CVF is suspected when the clinical and/or brain MRI features are suggestive of SIH but the spine MRI shows no extradural collection. When one performs decubitus myelograms, contrast density and timing are complementary factors that help capture the egress of contrast from a distended spinal meningeal diverticulum into adjacent paraspinal veins.9,24,25 Maneuvers such as resisted inspiration while scanning may accentuate the CVF due to dynamic relationships between subarachnoid and venous pressures.8,11,26 CVFs have been described more frequently on the right side of the spine than on the left, but there are no reliable ways to know on which side the CVF will occur; therefore, evaluation of both sides may be needed. Right and left myelograms on separate days were reported initially,27,28 while a same-day technique was later reported.12 Additional tips that some authors have reported useful for decubitus CTM are to perform >1 phase of scanning or to use real-time bolus-tracking to improve detection.29,30 Decubitus DSM and CTM both have unique advantages for CSF leak detection. While DSM has higher spatial and temporal resolution (Fig 4), CTM provides cross-sectional detail, has better contrast resolution, and is less susceptible to motion artifacts in nonsedated patients.
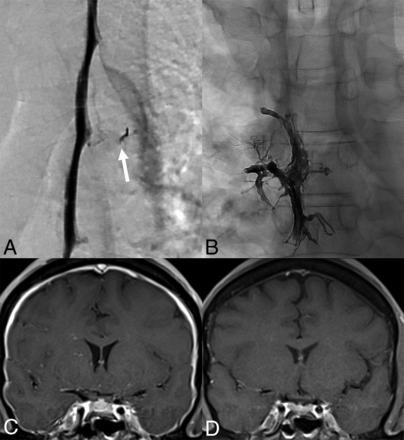
CVF on a DSM (A) in a 47-year-old man with a right T5 CVF (arrow) that was treated with Onyx (Medtronic) embolization (B). The pre-embolization brain MRI (C) demonstrates dural enhancement that nearly normalized 1 month after embolization (D).
Conebeam CT myelography (CB-CTM) can be a helpful adjunct to DSM for CVFs or lateral dural tears.31,32 The primary advantage of CB-CTM, when used in conjunction with DSM, is that it permits high-resolution cross-sectional imaging with a minimal delay between contrast injection and image acquisition. This can be helpful to clarify indeterminate findings on DSM, capitalizing on the advantages of both modalities (Fig 5). Dual-energy CT and MRI with intrathecal gadolinium were also evaluated for CSF leaks.33,34
Benefit of conebeam CT for detection of a right T6 CVF. Select unsubtracted (A) and subtracted (B) images from a right lateral decubitus DSM show faint linear paraspinal venous opacification (A, arrow). The finding is extremely difficult to appreciate on the unsubtracted image and essentially not seen on the subtracted image (B) due to a combination of pulmonary markings and respiratory motion. Axial (C) and coronal (D) MIP images from a conebeam CT performed minutes later during active contrast injection show a clear right T6 CVF involving several lateral branch veins. In cases such as this, conebeam CT serves as an excellent adjunct to DSM.
Advances in Treatment
The greatest nonsurgical innovations for CVF have been neuroradiologist-led treatments with fibrin occlusion and embolization, which were published in May 2021 and are now practiced throughout the world.
Historically, epidural patching for CVFs was not successful, but it was difficult to understand why because many details were not clearly discussed, including the image-guidance technique, the volume and type of injectate (fibrin glue or autologous blood), whether contrast was mixed with the injectate, and, most important, whether the patch was performed along the CVF course or rather in the dorsal epidural space. CT-guided fibrin glue occlusion was successfully performed at a single center with effective results.35,36 Subsequently, a multi-institutional and international study was performed, in which 59.7% of patients had complete clinical improvement and 34.5% had partial clinical improvement, with corresponding brain MRI improvement. One statistically significant conclusion from this study was that clinical improvement was observed if the injectate spread was concordant with the CVF drainage pattern (Fig 6).37
CVF occlusion with targeted fibrin glue patching. A, Axial right decubitus CTM shows a right T7 CVF with a paravertebral segmental vein (arrows). B, Axial CT treatment image demonstrates injected fibrin glue within the neural foramen and paravertebral space that matches the CVF drainage course, which is an important feature for treatment success. Posttreatment axial right decubitus CTM (C) shows resolution of the CVF. The pretreatment brain MRI (D) demonstrates dural enhancement (arrows) that resolved 1 month after patching (E).
Spinal CVF embolization is a novel technique in which the paraspinal vein is catheterized and a liquid embolic is injected, resulting in a cast, thereby occluding the CSF-venous connection (Fig 4). It was first described at a single center with effective results clinically and on brain MRI and was subsequently published in larger studies.7,38,39 Besides the success with CVF embolization, the transvenous technique led to greater interest and understanding of the paraspinal venous system, which was not well-recognized. An atlas of the venous anatomy and guidance on where to approach the CVF throughout the spine have provided substantial guidance to the operating physician.40
While both fibrin occlusion and embolization have markedly transformed spinal CSF leak treatment, surgery serves a vital role in patients with chronic dural tears and refractory CVFs and can be performed after a minimally invasive technique is attempted.4,41
The Promising Technologic Future
Photon-counting detector CT (PCD-CT) will potentially serve an integral role in spinal CSF leak detection. Compared with traditional energy-integrating detector (EID) CTs, PCD-CT demonstrates higher spatial and temporal resolution, along with inherent spectral sensitivity, which can aid in detecting subtle CSF leaks without the need for dual-energy/dual source techniques. In the context of spinal CSF leaks, CVFs were first evaluated by PCD-CT and showed a high diagnostic yield.13,42 One promising application is for CVFs that demonstrate drainage in the internal epidural venous plexus, an area that can be overlooked on myelography, given the challenges in spatial resolution.43 High spatial resolution is also beneficial for detecting subtle venous opacification adjacent to meningeal diverticula and vertebral elements, where the juxtaposition of high-attenuation structures is difficult to resolve on EID-CT (Fig 7). In addition to CVFs, PCD-CT has been shown to aid in the detection of CSF leaks from dural tears, which also demand rapid scanning and high spatial resolution.44 The high spatial resolution of PCD-CT can be maximally leveraged using sharper quantitative kernels in image reconstruction, which can result in increased image noise. A deep learning algorithm has been applied to denoise PCD-CT images to enhance the diagnostic quality, permitting the use of sharper kernels while retaining an acceptable SNR ratio (Fig 8).45 As the technology becomes more widespread, PCD-CT will undoubtedly showcase additional benefits in CSF leak detection.
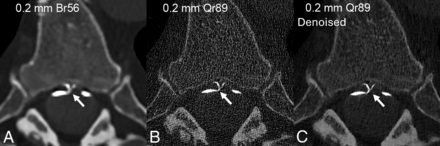
The advantage of high spatial resolution to detect a right T10 CVF on photon-counting CTM. Axial and sagittal 0.2-mm images (A and B) from a right decubitus photon-counting CTM reconstructed using a relatively smoother Br56 kernel, demonstrate a right T10 CVF involving the ventral and dorsal internal epidural venous plexus (A and B, arrows). Axial 0.2-mm images at the same level, reconstructed using both a smoother Br56 kernel (C) and a sharper Qr89 kernel with denoising (D), show involvement of the intervertebral vein that is only evident on the sharper Qr89 kernel. In some cases, maximizing spatial resolution using a sharper kernel with denoising is necessary to appreciate the full extent of venous opacification.
Use of denoised sharp kernel images on photon-counting CTM for localization of a T5 ventral dural tear. Axial images from a prone dynamic photon-counting CTM, all at the same slice, time point, and window/level setting, demonstrate a ventral dural tear just to the right of midline (A–C, arrows). The precise location of the ventral leak is demonstrated with greater resolution when using a sharper Qr89 kernel (B and C) compared with a smoother Br56 kernel (A). Because the Qr89 kernel introduced noise into the image, a denoising algorithm was applied to permit the use of a sharper kernel while retaining an acceptable SNR (C).
While there are many known clinical and imaging features of SIH, there are also many unknowns, including the underlying pathophysiologic state and when a spinal CSF leak will lead to symptoms and abnormal imaging findings. The brain MRI findings can occasionally be normal in the setting of a spinal CSF leak.46 Studies on MR elastography and deep learning have shown promise in detecting underlying SIH in this context. In a pilot study, MR elastography found distinct stiffness and damping ratio patterns in SIH relative to controls.47 While other brain MRI scoring systems evaluate the surface of the brain and downward morphologic sagging of the brainstem, MR elastography can evaluate changes in the brain parenchyma. In another study, an internally validated deep learning algorithm was created to identify patients with a CSF leak on the basis of brain MR findings.48 Additionally, glymphatic flow has been recently investigated in the setting of CVFs. A small case series demonstrated that patients with SIH may have impaired glymphatic clearance, which could be restored after successful treatment.49 These studies provide a glimpse into how advanced imaging techniques may provide increased sensitivity for the detection of SIH in patients who might have otherwise been inappropriately dismissed because of conventionally normal brain MRI findings, as well as provide insight into the pathophysiologic underpinnings of spinal CSF leaks.
Redefining and Reaffirming Doctrines
The Monro-Kelli doctrine posits that the combined volume of neuronal tissue, blood, and CSF is constant within the rigid skull and that any increase or decrease in one of these elements will lead to a reciprocal change in the others to maintain intracranial pressure homeostasis. However, studies have found that the calvaria in SIH may grow inward along the inner table of the skull, resulting in layered hyperostosis, challenging the premise of this doctrine.50,51 Conversely, the doctrine has been reaffirmed in the postsurgical setting in new ways so that in patients with surgical closure of a dural leak, ventricular CSF will increase with concomitant decrease in SIH brain findings.52
After the discovery of CVFs, it became apparent that they most commonly occur in the thoracic spine but rarely can occur in the lumbar and cervical spine. The revelation that sacral CVFs may also exist has added a new diagnostic area for evaluation.53
Spontaneous intracranial hypotension can be a misnomer, because the opening pressure in many patients is within the normal range. In fact, pressures can even be elevated, particularly in patients with obesity.54 This knowledge has diminished the role and need for opening pressure measurements for establishing a diagnosis of SIH.
Spinal and skull base CSF leaks are occasionally and erroneously categorized together, because it has been well-established that spinal CSF leaks are associated with SIH, and skull base leaks, with intracranial hypertension. This distinction has been recently reaffirmed in an analysis of skull base leaks while acknowledging that skull base leaks may rarely result in SIH.55,56
Redefining the Neuroradiologist
Spinal CSF leak care is a multidisciplinary effort. Various physician specialties, midlevel providers, nurses, technologists, and patient coordinators all contribute to the patient experience. While the neuroradiologist has always been intimately involved in this care team, the neuroradiologist’s roles have moved to the forefront. Because SIH is often first suggested on brain MRI and because myelography is essential to precise spinal CSF leak diagnosis, it has been the neuroradiologist who has led the effort to optimize the positioning techniques, scanning parameters, radiation safety, and new technologies to find the often-elusive spinal leak. These have transformed patients’ lives and our profession. Due to the advances in patching and embolization, the neuroradiologist has served a more integral role as a treating physician rather than a diagnostic physician. Neuroradiologists have embraced these new roles with dedicated clinics, where in certain CSF leak centers, the neuroradiologist is the primary physician contact for a patient with a CSF leak. While direct patient care is not new for neuroradiologists, these new avenues in CSF leak care have certainly expanded their broad armamentarium.
Patient-Centered Care
Diagnosis and treatment of spinal CSF leaks can occasionally be challenging. The entity is not well-understood, is underdiagnosed, and has many clinical mimics.57,58 Some patients are often dismissed and labeled incorrectly as having chronic pain or even psychiatric diagnoses, which can leave patients dejected and losing trust in the medical system. Thus, many patients join support groups to seek advice from others with similar symptoms and to ultimately gain hope of recovery. Spinal CSF leak providers have recognized the patient role in this condition and have routinely embraced patient participation at their conferences, where there have been physician and patient sessions to unite the two. Moreover, this participation converged at the inaugural “Bridging the Gap” conference in 2023, where patients shared their experiences, frustrations, and joys of their spinal leak journey.59 These unique experiences have both strengthened the physician-patient relationship and, by providing a platform for patients to share their experiences, refined the diagnostic acumen of treating clinicians.
Our Job is Not Finished
While we are proud of these major developments in this spinal CSF leak era, there is much to be discovered, and many questions are left unanswered. To date, research studies are retrospective and largely from individual institutions, which may be difficult to replicate at other centers with different resources. Prospective study designs, clinical trials, and comparative studies are needed to elevate spinal CSF leak care to new levels.60 Furthermore, we need more specific clinical tests and scoring systems that are germane to the diagnosis to better triage which patients should undergo further work-up. These can serve as an adjunct to existing imaging scoring tests.61,62 Last, we need to increase awareness of this disease. Patients with spinal CSF leaks can enter the medical system through different venues and various physicians, necessitating understanding of the main facets of this entity for prompt diagnosis and treatment.
Conclusions
Spinal CSF leak care has many major accomplishments in diagnosis, treatment, and the patient experience. The neuroradiologist has played an integral role in its transformation, and journals like the AJNR serve as a beacon for disseminating these breakthroughs.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
- 8.
- 9.
- 10.
- 11.
- 12.
- 13.
- 14.
- 15.
- 16.
- 17.
- 18.
- 19.
- 20.
- 21.
- 22.
- 23.
- 24.
- 25.
- 26.
- 27.
- 28.
- 29.
- 30.
- 31.
- 32.
- 33.
- 34.
- 35.
- 36.
- 37.
- 38.
- 39.
- 40.
- 41.
- 42.
- 43.
- 44.
- 45.
- 46.
- 47.
- 48.
- 49.
- 50.
- 51.
- 52.
- 53.
- 54.
- 55.
- 56.
- 57.
- 58.
- 59.
- 60.
- 61.
- 62.
- © 2024 by American Journal of Neuroradiology