Abstract
BACKGROUND AND PURPOSE: Flat-panel detector CT immediately after mechanical thrombectomy can detect complications, including early hemorrhagic transformation and subarachnoid hyperdensities. The clinical significance of subarachnoid hyperdensities in patients undergoing mechanical thrombectomy remains unclear.
MATERIALS AND METHODS: We studied 223 patients who underwent mechanical thrombectomy for anterior circulation stroke who had flat-panel detector CT performed immediately after the procedure and had follow-up imaging within 24 hours. Subarachnoid hyperdensity severity was categorized into 5 grades (subarachnoid hyperdensities, 0: absent to subarachnoid hyperdensities, IV: extensive). Baseline and procedural characteristics as well as outcome measures were analyzed using group comparisons and multivariable logistic regression analyses.
RESULTS: Overall, 100/223 (45%) patients showed subarachnoid hyperdensities on immediate postinterventional flat-panel detector CT. The factors associated with an increased subarachnoid hyperdensity risk were the following: medium-vessel occlusion or distal-vessel occlusion compared with a large-vessel occlusion, a more distal device position, a higher number of device passes, a larger volume of contrast applied, worse final reperfusion expanded TICI, and after receiving IV thrombolysis. The occurrence of subarachnoid hyperdensity grades II–IV was independently associated with worse functional outcomes (adjusted OR for mRS, 3–6: 2.2; 95% CI 1.1–4.3), whereas patients with subarachnoid hyperdensity grade I had outcomes similar to those in patients without subarachnoid hyperdensities.
CONCLUSIONS: Our study identified risk factors for subarachnoid hyperdensities, most of which reflect increasingly challenging procedures or more peripheral recanalization attempts. The presence of subarachnoid hyperdensity grades II–IV was associated with poorer outcomes, suggesting the need for personalized strategies to reduce its incidence and severity or potentially improve recovery after subarachnoid hyperdensities.
ABBREVIATIONS:
- aOR
- adjusted OR
- DVO
- distal-vessel occlusion
- eTICI
- expanded TICI
- FDCT
- flat-panel detector CT
- LVO
- large-vessel occlusion
- MT
- mechanical thrombectomy
- MVO
- medium-vessel occlusion
- SH
- subarachnoid hyperdensities
SUMMARY
PREVIOUS LITERATURE:
Flat-panel detector computed tomography (FDCT) has gained popularity for its rapid–high-resolution imaging capabilities at disposal in the angiosuite. FDCT can reveal imaging findings occult on conventional DSA–the significance of which remains a matter of interest–especially in the setting of endovascular stroke treatment. SH is one such findings and may be due to true hemorrhage or extravascular contrast medium. Existing literature has addressed newly detected hyperdensities in various brain compartments on CT after endovascular treatment–focusing on their prognostic value and outcome or aiming at differentiating blood from contrast. However–studies focusing on newly detected SH on immediate post-procedure FDCT are still scarce.
KEY FINDINGS:
Increased risk of SH on immediate FDCT is seen with MVOs/DVOs–multiple thrombectomy attempts–more distal device position–larger contrast volume used–lower reperfusion scores–and intravenous thrombolysis. Patients with SH grades II-IV had worse outcomes–while patients with SH grade I had similar outcomes compared to patients without SH.
KNOWLEDGE ADVANCEMENT:
Our study identified risk factors for SH on FDCT after MT in patients with acute anterior circulation ischemic stroke–primarily related to the complexity of the intervention. SH grades II-IV were associated with worse outcomes–highlighting the potential need for more intensive post-interventional monitoring and individualized treatment strategies.
Mechanical thrombectomy (MT) has become the standard of care for patients with acute ischemic stroke due to large-vessel occlusion (LVO).1,2 Early follow-up imaging studies have identified several complications associated with MT, including arterial vasospasm, dissection, perforation, and re-occlusion.3 Other postinterventional imaging findings include (progressive) infarct demarcation, distal embolization in new territories, hemorrhagic transformation, and subarachnoid hyperdensities (SH).3 In recent years, the use of flat-panel detector technology has attracted widespread interest within the neuroradiology community. Flat-panel detector CT (FDCT) allows the rapid acquisition of high-resolution images, almost matching the quality of conventional multidetector CT, and is increasingly used during various neurointerventional procedures.4⇓⇓⇓-8 FDCT acquired during or immediately following MT may reveal imaging findings that are occult on conventional DSA. FDCT is available directly in the angiosuite, eliminating the need for patient transfer to a CT suite. If incomplete reperfusion or peri-interventional complications are detected directly in the angiosuite, this may allow performing additional interventions such as secondary MT, intra-arterial lytics, or timely rescue maneuvers and may potentially be important for immediate postinterventional patient care (eg, blood pressure management or antithrombotic regimen). SH are one such finding and may be related to hemorrhage or contrast staining.9 However, the prognostic value of SH in patients undergoing MT remains unclear.
There is a growing body of literature dealing with newly detected hyperdensities in different brain compartments seen on CT after endovascular treatment.9⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓-28 The literature mainly focuses on the prognostic value and outcome and/or aim to differentiate blood from contrast staining.9⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓-28 However, studies concentrating on newly detected SH on FDCT performed immediately after MT for acute stroke are still scarce.29 Hence, our goal was to investigate the incidence of SH on immediate postprocedural FDCT, identify risk factors for SH, and evaluate the impact of SH on short-term clinical outcome.
MATERIALS AND METHODS
Patient Population
All consecutive patients with acute ischemic stroke who were treated with MT between July 2020 and December 2022 at our tertiary-level center were retrospectively identified from the stroke database. For inclusion in the present analysis, patients had to meet the following criteria: 1) anterior circulation acute ischemic stroke, 2) FDCT performed immediately following the intervention, and 3) follow-up imaging (CT or MR imaging) within 24 hours. Initially, the decision to obtain postinterventional FDCT was at the discretion of the neurointerventionalist. FDCT tended to be performed more frequently in cases involving more complicated procedures characterized by multiple maneuvers, distal thrombectomies, tandem occlusions, the need for antiplatelet therapy during emergency stent placement, intracranial stenosis, peri-interventional dissection in the cervical vessel, the potential administration of adjunctive intra-arterial lytics, and the exclusion of bleeding or other potential complications. However, after the initial phase, all institutional neurointerventionalists systematically obtained FDCT after every acute stroke procedure.30 This study adhered to the principles outlined in the Declaration of Helsinki and was approved by the local ethics committee (reference ID 231/14, 2019–00547, 2023–00892).
Clinical Data
The clinical data were retrospectively extracted from the prospective stroke database and the electronic medical charts. These included age, sex, stroke risk factors (hypertension, diabetes mellitus, hyperlipidemia, coronary heart disease, current smoking, atrial fibrillation), medications at baseline (antihypertensives, anticoagulants, antiplatelets, or lipid-lowering medications), baseline NIHSS score, NIHSS score at 24 hours, prestroke mRS, mRS at 90 days, and administration of IV thrombolytics. Distal intracranial ICA and M1 occlusions were defined as LVO; M2, A1, and A2, as medium-vessel occlusion (MVO); and M3 and A3, as distal-vessel occlusion (DVO).31 MVO and DVO were treated within ongoing trials or after an interdisciplinary discussion based on the severity of focal neurologic deficits, vessel occlusion, IV thrombolysis eligibility, and technical considerations such as vessel tortuosity and diameter.
All procedures were performed with the patient under general anesthesia using a stent retriever, a direct aspiration catheter, or a combination of the two. Procedural characteristics of the MT, such as the volume of contrast used during the procedure (milliliter), number of maneuvers/passes, and the most distal catheter position (M1, M2, M3, M4, A1, A2, or A3) were registered. Active extravasation seen on DSA was also documented. Reperfusion outcomes were assessed using the expanded TICI (eTICI) scale, which ranges from 0, indicating no reperfusion, to 3, indicating complete reperfusion (100%) of the target downstream territory. Intermediate grades, 2a, 2b50, 2b67, and 2c reflect reperfusion levels within the target downstream territory of 1%–49%, 50%–66%, 67%–89%, and 90%–99%, respectively.32
Imaging Analysis
Baseline imaging (CT or MR imaging), postprocedural FDCT after MT, and early follow-up imaging (CT or MR imaging) within 24 hours after the procedure were analyzed by a board-certified radiologist (B.L.S.).
Baseline and early follow-up CT included NCCT, CT angiography of the intra- and extracranial arteries, and mostly CT perfusion. Baseline and early follow-up MR imaging studies were performed at a magnetic field strength of 1.5T or 3T. Stroke protocols mostly included axial DWI and a matching ADC map, axial FLAIR, 3D TOF-MRA of the intracranial arteries, axial SWI, 3D contrast-enhanced T1WI, 3D contrast-enhanced MRA of the intra- and extracranial arteries, and MR perfusion.
The Sine Spin FDCT (ARTIS icono biplane; Siemens) represents the latest generation of cone-beam CT. It uses a dual oblique path for image acquisition aimed at minimizing artifacts and optimizing soft-tissue brain imaging.33 FDCT was acquired according to the “7sDCT Sine Spin protocol,” details of which have been described previously.33
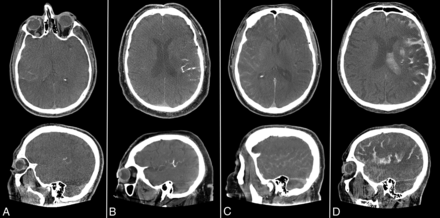
The baseline imaging (CT or MR imaging) studies were reviewed for the intracranial occlusion site (distal intracranial ICA, M1, M2, M3, A1, A2, or A3). FDCT studies were evaluated for the presence of SH (yes/no). SH were graded (I–IV) as follows: grade I, hyperdensities in 1 or 2 adjacent sulci; grade II, hyperdensities in ≥3 adjacent sulci but confined to a single lobe; grade III, diffuse sulcal hyperdensities affecting ≥2 lobes; and grade IV, diffuse sulcal hyperdensities affecting ≥2 lobes and intraventricular extension or extension into basal cisterns (Fig 1). Grading was independently performed by 2 board-certified radiologists (T.D. and B.L.S.), and disagreements were settled by consensus discussion. If SH were evident on FDCT, the course was reviewed on follow-up imaging (ie, complete resolution, similar expansion, marked reduction, or clear progression).
Examples of SH grades I to IV. A, SH grade I with hyperdensities in 2 neighboring sulci. B, SH grade II with hyperdensities in >2 neighboring sulci but confinement to 1 lobar area. C, SH grade III with diffuse sulcal hyperdensities affecting >2 lobes. D, SH grade IV with diffuse hyperdensities affecting >2 lobes and intraventricular extension.
The ASPECTS was assessed at baseline imaging. If patients underwent CT for baseline imaging, ASPECTS was calculated automatically using CINA-ASPECTS, Version 1.4.2.0 (Acivenna.AI), a cloud-based CE-marked deep learning algorithm integrated into an AI orchestration suite (Calantic, Version 1.2.0; Bayer). When automated scoring failed or when patients underwent MR imaging, ASPECTS was scored by a board-certified radiologist. ASPECTS is a 10-point score used to evaluate early ischemic changes in the hypoperfused area of the MCA. A score of 10 indicates the absence of such changes, and 1 point is subtracted for each standardized brain region involved. For the MR imaging–based ASPECTS assessment, DWI was used, and a region had to have a diffusion abnormality in ≥20% of its volume to be considered positive for early ischemic changes.34
Readers were blinded to the outcome but not to the patient’s medical history.
Statistical Analysis
Descriptive statistics are presented as frequencies and percentages for categoric variables and median with interquartile range for continuous variables. Continuous variables were evaluated using the Student t test or the Wilcoxon-Mann-Whitney U test, whereas categoric variables were evaluated using the Fisher exact test or the χ2 test. For >2 groups, comparisons for continuous variables were completed using either ANOVA or the Kruskal-Wallis test. Interrater agreement for different SH categories (0 to IV) is reported as a Cohen κ. Multivariable logistic regression was performed to elucidate the association of selected factors (age, sex, NIHSS on admission, IV thrombolysis, number of maneuvers, prestroke medication [anticoagulant and antiplatelet], site of the occlusion, and the most distal site of recanalization) on which group of SH was seen (SH 0 versus SH grades I–IV).
The association of different SH categories (0 to IV) on several outcomes such as the mRS score at 3 months, dichotomized mRS (0–2 versus 3–6), NIHSS at 24 hours, early neurologic deterioration (defined as a change in NIHSS of ≥4 between admission and 24-hour follow-up), and mortality at 3 months (equivalent to mRS 6) was modeled. Ordinal logistic regression was used for the mRS shift analysis. The NIHSS at 24 hours was assessed using quantile regression at the 50% quantile (corresponding to the median), whereas binary outcomes were assessed using logistic regression.
Analyses using inverse probability weighting of the association of different SH categories on clinical outcomes were adjusted for the following covariates: age, sex, admission NIHSS, occlusion site, baseline ASPECTS, IV thrombolysis, prestroke mRS, parenchymal hyperdensities seen on FDCT, and the final eTICI. Those factors were based on the most robust predictors of functional outcome after stroke.35 Due to the limited sample size of SH IV (11 cases), the model correction was found to be unstable. Therefore, only a reduced set of variables (age, sex, admission NIHSS, baseline ASPECTS, IV thrombolysis, and parenchymal hyperdensities on FDCT) could be included in the analysis for the comparison between SH 0 and SH IV. Results are presented as adjusted ORs (aORs) with 95% CIs for the mRS analyses and logistic regression and as regression coefficients with 95% CIs for the NIHSS variables. Adjusted regressions were performed with both complete cases and imputation of missing data. Patients who had died at 3 months were assigned an mRS score of 6 and an NIHSS score of 42. The reported P values were not adjusted for multiple comparisons, which should be considered when interpreting the results.
All statistical analyses were performed with R statistical and computing software, R 4.3.136 (http://www.r-project.org/) and/or Stata 17.0 (StataCorp).
RESULTS
Cohort
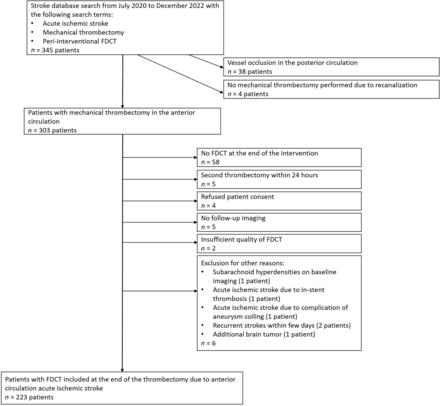
Between July 2020 and December 2022, two hundred twenty-three patients, of whom 113/223 (50.7%) were women (median age, 75.5 years; interquartile range, 63.3–83.1 years) met the inclusion criteria (Fig 2). This represents approximately one-third of all patients undergoing anterior circulation MT at our tertiary center during this period. Throughout the study period, individuals with FDCT tended to have a lower prestroke mRS, a higher prevalence of MVOs or DVOs, lower ASPECTS on baseline imaging, an increased frequency of symptom onset either unknown or observed on wake up, and a higher 24-hour NIHSS in contrast to patients for whom no FDCT was acquired (Online Supplemental Data).
Flow chart depicting patient selection process.
Presence and Subtypes of SH
Postprocedural FDCT revealed SH (grades I–IV) in 100 cases (45%). Two FDCTs were of insufficient quality to identify and classify SH due to extensive beam-hardening artifacts and aliasing artifacts, respectively (Fig 2). Forty patients had SH grade I; 26 patients, grade II; 23 patients, grade III; and 11 patients, grade IV. The first-line technique at our tertiary center is combined stent retriever and distal aspiration thrombectomy. Seven patients (7/223, 3%) were treated with an aspiration catheter alone. Two patients (2/7, 29%) had SH (both grade I). Interrater agreement was very good (Cohen κ. 0.9; 95% CI, 0.86–1.00). The evolution of SH on follow-up imaging differed among groups (P = .004; Online Supplemental Data), with more severe SH grades (II–IV) less often showing complete resolution within 24 hours.
Factors Associated with Occurrence of SH
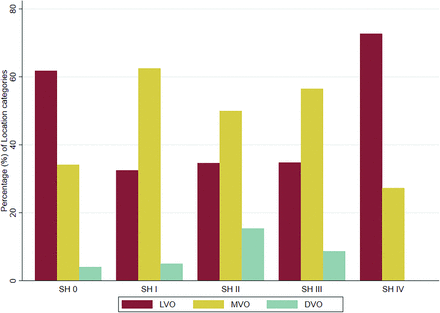
Most baseline factors were similar between patients with and without SH, as shown in Table 1. Baseline characteristics stratified by all SH groups are shown in the Online Supplemental Data. There was a difference in the baseline occlusion site between the groups with and without SH, with overall MVO and DVO occurring more frequently (62% versus 38%, P < .001; Table 1 and Fig 3) in patients with SH I–IV.
Baseline intracranial occlusion site (LVO, MVO, and DVO) stratified by SH.
Baseline characteristics stratified by SH seen on FDCT
Analysis of procedural characteristics found that patients with SH grades I–IV had a higher number of thrombectomy passes (2 [1–4] versus 1 [1–2]; P < .001), more often had medium (68% versus 59%) or distal vessels (28% versus 17%; P < .001) as the most distal microcatheter sites, were less likely to have higher eTICI scores (P = .014) and received more contrast during the procedure (133 [100–190] mL versus 110 [90–160] mL; P = .035) than patients without SH (Table 2). The incidence of active extravasation seen on DSA increased at higher SH grades (SH I, 0/40 [0%]; SH II, 1/26 [4%]; SH III, 4/23 [17%]; SH IV, 8/11 [73%]; P < .001; Online Supplemental Data). There was also a trend toward a higher rate of IV thrombolysis in patients with SH (52.0% versus 39.8%, P = .07; Table 1), which reached significance when all SH groups were stratified separately (P = .019, Online Supplemental Data).
Procedural characteristics and outcome measures stratified by SH seen on FDCT
The findings of the multiple logistic regression analysis indicated elevated odds of presenting with SH grades I–IV in patients with more thrombectomy passes (OR per pass, 1.5; 95% CI, 1.2–1.9) and after receiving IV thrombolysis (OR, 2.0; 95% CI, 1.1–3.8). When we examined the most distal device site, location in medium (OR, 3.8; 95% CI, 1.2–12.3) or distal vessels (OR, 4.5; 95% CI, 1.2–16.9) was associated with higher odds of presenting with SH. A similar tendency was observed for patients with a baseline occlusion site classified as MVO or DVO (OR, 1.8; 95% CI, 0.9–3.5).
Functional Outcome
In the unadjusted analyses, we sought to identify potential associations of SH with several outcomes. When we compared patients without SH separately with every SH group individually, the presence of the worst grade (IV) was associated with higher odds (3.9; 95% CI, 1.1–13.9) of experiencing early neurologic deterioration. In the next step, grouping individuals with similar point estimates and hence comparing SH II–IV with SH 0–I groups was associated with worse functional outcomes (ordinal shift: OR, 1.9; 95% CI, 1.1–3.4; dichotomized mRS: 3–6; OR, 2.0; 95% CI, 1.0–3.8) and higher mortality (OR, 2.1; 95% CI, 1.1–4.2) in the SH II–IV groups.
In the adjusted analyses, we observed a predominant tendency toward increasing point estimates, indicating higher odds of adverse outcomes in the SH II, III, and IV groups. The SH II group showed increased odds of a higher mRS at 90 days (aOR, 3.2; 95% CI, 1.4–7.4) and an increased risk of 90-day mortality (aOR, 3.7; 95% CI, 1.4–9.9) compared with the SH 0 group. In a next step, combining groups with similar point estimates and hence comparing SH II–IV with SH 0–I was associated with worse functional outcomes (ordinal shift: aOR, 2.1; 95% CI, 1.2–3.8; dichotomized mRS: 3–6; aOR, 2.2; 95% CI, 1.1–4.3) in the SH II–IV groups (Fig 4).
A, Distribution of mRS scores at 90 days for patients without SH and patients with SH I–IV on FDCT. B, The association of different grades of SH 0–IV with mRS at 3 months, mRS dichotomized (0–2 versus 3–6), and mortality (equivalent to mRS 6). Analyses were performed using multivariable ordinal/logistic regression, adjusting for prespecified confounders (see Materials and Methods).
The results of the unadjusted, adjusted, and grouped comparisons are shown in Fig 4 and the Online Supplemental Data, respectively.
DISCUSSION
Our study found that almost one-half of the patients undergoing MT due to anterior circulation occlusion showed SH on immediate postinterventional FDCT. An increased risk of SH is associated with MVOs and DVOs, multiple thrombectomy attempts, a more distal device position, a larger volume of contrast applied, lower reperfusion scores, and after receiving IV thrombolysis. Patients with SH grades II–IV had overall worse outcomes, whereas patients with SH grade I had an outcome comparable with that of patients without SH on FDCT.
A growing body of literature is addressing the topic of newly detected hyperdensities after MT. However, the studies vary widely regarding the timing of postprocedural imaging, the imaging technique (FDCT, CT, dual-energy CT, or MR imaging) and the precise location of hyperdensities (parenchymal, subarachnoid, or ventricular). In addition, the terminology is inconsistent and poorly defined, with terms ranging from contrast extravasation, hyperdensity, contrast enhancement, metallic hyperdensity, and contrast staining to hemorrhage.9⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓–29
Previous studies have mainly focused on parenchymal hyperdensities only14,22,25 or included hyperdensities in all compartments11,16,20 and reported frequencies ranging from 28% to 84%.11,14,16,20,22,25 Compared with the study by Parrilla et al20 from 2012, we found a higher incidence of SH in our cohort (12.5% versus 45%). This may be explained by evolving indications in MT targeting more distal occlusion sites as well as improved imaging quality with new-generation FDCT technology. A more recent study reported a prevalence of SH on FDCT of 37.1%, which is similar to that observed in our cohort.29
Performed either peri-interventionally or immediately postinterventionally, FDCT serves a purpose similar to that of postprocedural multidetector CT. However, FDCT has the advantage of being performed directly in the angiosuite, eliminating the need to transfer the patient to a CT suite. While multidetector CT and FDCT may provide comparable diagnostic performances,33 identification of incomplete reperfusion or peri-interventional complications on FDCT directly in the angiosuite may prompt consideration of additional treatments such as secondary MT, intra-arterial lytics, or timely rescue maneuvers.37⇓–39 Immediate identification of cerebral intraparenchymal hyperdensities after thrombectomy provides valuable prognostic information about the eventual infarct volume and may aid in the early prediction of patient clinical outcome and acute management after thrombectomy.40,41
Accurate and reliable differentiation between hemorrhage and contrast is essential to guide subsequent antithrombotic therapy. However, despite its advantages, FDCT obtained peri- or immediately postinterventionally faces challenges in reliably distinguishing between blood and extravascular contrast medium. Because contrast usually resolves within 24 hours, accurate differentiation is typically possible at 24-hour follow-up imaging. Although it is not yet a standardized method, dual-energy CT can accurately differentiate between blood and contrast.29 It offers a promising avenue for accurate blood/contrast differentiation. Emerging technologies such as photon-counting CT also show promise for improving blood/contrast differentiation in the future.42
In line with the study by Zidan et al,29 we also found that SH were more prevalent in MVOs and DVOs than in LVOs. In addition, distal device placement during MT increased the likelihood of presenting with SH grades I–IV. The advent of dedicated devices designed for distal MT has expanded the range of treatment options. The anatomic characteristics of smaller vessels, however, may render the procedure more challenging, requiring navigation of microcatheters and guidewires through numerous bifurcations. Also, vessel tortuosity may lead to displacement, straightening and stretching of smaller vessels.15,43 These complexities of device handling increase the likelihood of vessel damage, rupture of adjacent arterioles and venules, disruption of the BBB, and subsequent SH (Fig 5).15,29,43
Possible mechanism leading to SH. A, Ventrolateral view of the brain and circle of Willis. B, Close-up showing a proximal M2 occlusion with an inserted stent retriever. C, Close-up during the retrieval of the stent retriever. During navigation, smaller vessels (M2 and beyond) tend to straighten more than proximal vessels. The perforators are exposed to excessive forces during thrombectomy due to stretching and may be sheared off, leading to subtle extravasation, which is occult on standard DSA but may be detected on FDCT as SH, in this case, a subarachnoid hemorrhage. © Inselspital, Bern University Hospital, Department of Neuroradiology.
A greater number of passes was found to be associated with a higher risk of presenting with SH grades I–IV. This observation is consistent with previous research.29 The amount of contrast administered can serve as a surrogate for the duration and complexity of the procedure and the number of device passes; administration of a higher volume of contrast was also linked to an increased risk of SH grades I–IV.16 The increase in the incidence of SH with more thrombectomy attempts is likely due to a cumulative effect of the inherent risk of vascular injury with each revascularization attempt. Initially, there may be a minimal microperforation of the vessel wall or endothelial damage that compromises the BBB. However, with repeat passes and/or subsequent reperfusion, complications may be exacerbated.15,29
In addition, our analysis showed that lower eTICI scores were associated with an increased likelihood of developing SH. This association is likely indicative of more complex procedures with a greater number of passes. A potential mechanism other than the number of maneuvers may be involved, as residual clots associated with lower eTICI scores could also cause more damage to the vessel wall when interacting with stent retrievers or aspiration catheters.
Contrast staining and enhancement occur when the BBB becomes more permeable due to endothelial cell damage, resulting in the leakage of the contrast agent from the blood vessels into the extracellular spaces.16 Hemorrhagic lesions, on the other hand, are thought to be caused by an additional degradation of the basal lamina. This disruption results in the extravasation of cellular blood elements from the microvessels.44 Various studies have consistently reported that blood and contrast agents can be differentiated according to the time they take to disappear.9,23 Hyperdensities that disappear within 24 hours are considered to be contrast agent, because extracellular contrast is mostly cleared within 24–48 hours.9,23
Blood, on the other hand, degrades and remains visible on imaging for several days to weeks.9,23 In our cohort, evolution of the SH on follow-up imaging differed significantly between groups, with the predominant trend indicating that milder grades of SH were more likely to resolve on follow-up imaging within 24 hours. We hypothesize that complete resolution of SH on follow-up imaging within this 24-hour-window may be indicative of the presence of contrast media in the subarachnoid space, whereas comparable or visibly increased SH may correspond to the presence of additional blood components. Furthermore, cases with a substantial reduction of SH could potentially involve minimal blood components, or the follow-up imaging performed within 24 hours may have captured the clearing process of the contrast agent while it was still in progress. Only very few patients with SH (8/100, 8%) demonstrated a clear progression of SH on the follow-up imaging. A substantial proportion (37/100, 37%) of all SH had completely resolved on follow-up imaging within 24 hours, a percentage (39.4%) similar to that reported in previous research.29
In the analysis of outcomes, we observed that outcomes in the SH 0 and SH I groups were comparable, whereas patients with SH II–IV had generally worse outcomes. A small amount of hyperdensities (SH I) appear to have a minor impact on outcome. The course of smaller intracranial vessels (M2 and beyond) tends to straighten more than proximal vessels during microcatheter/wire navigation. The displacement becomes even more pronounced during retrieval. This change leads to a straightening of the loops of the peripheral cortical branches, resulting in excessive forces on the perforators, which may be sheared off during MT due to stretching (Fig 5). During this procedure, only the endothelial cells of the vessels are potentially affected, resulting in leakage of the contrast agent but not extravasation of cellular blood elements from the blood vessels. However, as the procedure becomes more complex, with more thrombectomy passes and more contrast used, the extent of hyperdensities increases, leading to worse outcomes. A plausible hypothesis is that the basal membrane of the vessels may be compromised in these cases, potentially contributing to the incorporation of hemorrhagic components.
The SH IV group appears to have some unique features. Overall, MVO and DVO occurred more frequently than LVO in the group consisting of all patients with SH I–IV. However, when we analyzed the baseline intracranial occlusion site of the SH IV group only, this group was characterized by a higher proportion of LVO compared with all other SH groups (Fig 3). In addition, in 8 of 11 cases (73%), an active extravasation of contrast was observed on DSA, meaning that this group experienced peri-interventional complications, usually requiring a rescue maneuver.
Although this study was not designed to draw conclusions about therapeutic implications, the results suggest that patients in the SH II–IV groups might potentially benefit from more intensive postinterventional monitoring and treatment regimens, such as stricter control of arterial blood pressure and adjustment of antithrombotic regimens.
This study has several limitations. First, its retrospective and monocentric design introduces inherent biases related to study design. Second, there was a selection bias toward those receiving FDCT, and patients receiving FDCT do not represent a random sample from all patients undergoing MT. Third, the lack of histopathologic correlates presents a challenge in accurately distinguishing between contrast medium and blood; however, we tried to mitigate this issue with spatial correlation between FDCT findings and scheduled follow-up imaging. Finally, the study included only patients with anterior circulation stroke, which may limit the generalizability of the findings.
CONCLUSIONS
SH on immediate postinterventional FDCT appears to be a frequent finding, appearing in about one-half of patients undergoing MT. The following factors were associated with an increased SH risk: MVO or DVO compared with a proximal LVO, a more distal device position, a higher number of device passes, a larger amount of applied contrast, worse final reperfusion scores, and after receiving IV thrombolysis. Patients with SH II–IV showed overall worse outcomes, indicating a potential need for more intensive postinterventional monitoring and tailored treatment strategies. Further research is necessary to optimize outcomes in this MT subpopulation by testing personalized preventive and recovery strategies.
Acknowledgments
We thank Ms Susan Kaplan for English language support and Ms Anja Giger, Department of Neurosurgery, University of Bern, Inselspital, for the illustration.
Footnotes
J. Kaesmacher and T. Dobrocky contributed equally to this work.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
- 8.
- 9.
- 10.
- 11.
- 12.
- 13.
- 14.
- 15.
- 16.
- 17.
- 18.
- 19.
- 20.
- 21.
- 22.
- 23.
- 24.
- 25.
- 26.
- 27.
- 28.
- 29.
- 30.
- 31.
- 32.
- 33.
- 34.
- 35.
- 36.
- 37.
- 38.
- 39.
- 40.
- 41.
- 42.
- 43.
- 44.
- Received January 3, 2024.
- Accepted after revision March 28, 2024.
- © 2024 by American Journal of Neuroradiology