Abstract
BACKGROUND AND PURPOSE: Understanding sex-based differences in patients with glioblastoma is necessary for accurate personalized treatment planning to improve patient outcomes. Our purpose was to investigate sex-specific differences in molecular, clinical, and radiologic tumor parameters, as well as survival outcomes in patients with glioblastoma, isocitrate dehydrogenase-1 wild-type (IDH1-WT), grade 4.
MATERIALS AND METHODS: Retrospective data of 1832 patients with glioblastoma, IDH1-WT with comprehensive information on tumor parameters was acquired from the Radiomics Signatures for Precision Oncology in Glioblastoma consortium. Data imputation was performed for missing values. Sex-based differences in tumor parameters, such as age, molecular parameters, preoperative Karnofsky performance score (KPS), tumor volumes, epicenter, and laterality were assessed through nonparametric tests. Spatial atlases were generated by using preoperative MRI maps to visualize tumor characteristics. Survival time analysis was performed through log-rank tests and Cox proportional hazard analyses.
RESULTS: Glioblastoma was diagnosed at a median age of 64 years in women compared with 61.9 years in men (false discovery rate [FDR] = 0.003). Men had a higher KPS (above 80) as compared with women (60.4% women versus 69.7% men, FDR = 0.044). Women had lower tumor volumes in enhancing (16.7 cm3 versus 20.6 cm3 in men, FDR = 0.001), necrotic core (6.18 cm3 versus 7.76 cm3 in men, FDR = 0.001), and edema regions (46.9 cm3 versus 59.2 cm3 in men, FDR = 0.0001). The right temporal region was the most common tumor epicenter in the overall population. Right as well as left temporal lobes were more frequently involved in men. There were no sex-specific differences in survival outcomes and mortality ratios. Higher age, unmethylated O6-methylguanine-DNA-methyltransferase promoter and undergoing subtotal resection increased the mortality risk in both men and women.
CONCLUSIONS: Our study demonstrates significant sex-based differences in clinical and radiologic tumor parameters of patients with glioblastoma. Sex is not an independent prognostic factor for survival outcomes and the tumor parameters influencing patient outcomes are identical for men and women.
ABBREVIATIONS:
- EOR
- extent of resection
- FDR
- false discovery rate
- GTR
- gross total resection
- IDH1-WT
- isocitrate dehydrogenase-1 wild-type
- KPS
- Karnofsky performance score
- MGMTp
- O6-methylguanine-DNA-methyltransferase promoter
- MICE
- Multiple Imputation by Chained Equations
- STR
- subtotal resection
- US
- United States
- WHO
- World Health Organization
SUMMARY
PREVIOUS LITERATURE:
Understanding sex-based differences in patients with glioblastoma has been burning interest across the brain cancer society. Studies showing female survival advantage in this patient population are prominently known, however there are significant number of studies from across various regions of the world showing contradicting sex-based differences survival outcomes. Understanding sex-specific tumor parameter differences, especially in radiologic features across patients with isocitrate dehydrogenase-1 wild-type (IDH1-WT) glioblastoma has been limited. Therefore, there is need to understand sex-based differences in heterogenous glioblastoma population based on current World Health Organization (WHO) classification and with a focus on radiologic parameters.
KEY FINDINGS:
Radiomics Signature for Precision Oncology in Glioblastoma consortium data of grade 4, IDH1-WT, glioblastoma patients show significant sex-specific differences in age at diagnosis, preoperative Karnofsky performance score, tumor location, and volume distribution but not in survival rates. Additionally, factors determining the survival outcomes in males and females are similar.
KNOWLEDGE ADVANCEMENT:
Understanding the role of sex in glioblastoma outcomes is a crucial step toward personalizing treatment regimens across patients. We look at a large cohort of glioblastoma patients based on 2021 WHO classification and show no significant survival advantage based on sex. Therefore, our studies warrant for a closer inspection into sex-based differences in this patient population to efficiently determine the role of sex.
In recent years, there has been a growing interest in understanding the impact of patient sex on various cancers including glioblastomas.1,2 Several studies have shown associations between patient sex and various clinical and radiologic parameters in glioblastomas. For example, men have a 1.6 times higher incidence of grade 4 gliomas compared with women,1,3 a higher proportion of women have methylated O6-methylguanine-DNA-methyltransferase promoter (MGMTp), while preoperative Karnofsky performance score (KPS) and surgical techniques have not shown significant disparities.4
Most of the large population-based data sets used for the sex-based studies are based on older World Health Organization (WHO) classifications and include all grade 4 gliomas as glioblastomas.4⇓⇓-7 These data sets also lack detailed radiologic data to perform dedicated analysis of tumor morphology.4⇓-6 The 2021 WHO classification refined glioblastoma to include grade 4 gliomas with the isocitrate dehydrogenase-1 wild-type (IDH1-WT) gene.8 Few of the prior survival studies on sex-based differences focus on IDH1-WT grade 4 gliomas, however, the sample sizes are limited.9,10 None of the studies focusing on radiologic parameters adhere to the new glioblastoma definition.11⇓-13 Most of the prior literature also consists of single institution and/or single geographic location data with a predominant focus on United States (US)-based population, introducing an inherent risk of over- or underrepresenting a particular population, resulting in potentially skewed results.14⇓-16
To assess the impact of sex on clinical, radiologic, and survival parameters in grade 4 IDH1-WT glioblastoma, we used a recently compiled international and multi-institutional Radiomics Signatures for PrecisiON Diagnostics (ReSPOND) consortium data set. Post-2021 WHO classification of brain tumors, this is the largest study to investigate sex-specific differences in tumor parameters, including radiologic findings, survival outcomes, and mortality rates in glioblastomas. Overall and sex-based differences in patients with glioblastoma in the US compared with non-US regions are also explored.
MATERIALS AND METHODS
Population
This retrospective institutional review board–approved study included 1832 patients with glioblastoma, IDH1-WT, grade 4 from the ReSPOND consortium. After meeting the inclusion criteria, 1820 patients were included in the final analysis (Fig 1). ReSPOND is a growing international grade 4 glioma repository including data sets from multiple institutions across the United States, Europe, and Asia.17 Fifteen institutions/projects contributed the data for this analysis: University of Pennsylvania, Washington University School of Medicine in St. Louis, University of Pittsburgh Medical Center, Case Western Reserve University and University Hospitals, The Cancer Imaging Archive, King’s College London, New York University, Thomas Jefferson University, Henry Ford Health System, Ivy Glioblastoma Atlas Project, Ohio State University, Catalan Institute of Oncology, Yonsei University Health System, and publicly available data sets (ACRIN-FMISC, CAPTAC-GBM, and BraTS).
Description of total data and exclusion criteria.
The 2021 WHO classification of primary CNS tumors has recognized IDH1-WT gliomas as glioblastoma and IDH1-mutant gliomas as astrocytomas or oligodendroglioma (if 1p/19q co-deletion is present).8 In this study, only grade 4 IDH1-WT gliomas were included, which are now known as glioblastoma.
Spatial Distribution Atlases
Image Processing.
T1-weighted, T1-weighted post-gadolinium, T2-weighted, and T2-weighted FLAIR images with variable section thickness were acquired for all the patients. All the MR images were registered to a common anatomic template of the brain and interpolated to 1 mm3 isotropic resolution.18 Image processing, including registration, skull-stripping, and tumor segmentation was performed according to the BraTS preprocessing protocol18⇓-20 with the Cancer Imaging Phenomics Toolkit.21 Segmentations included enhancing region, necrosis region, and edema of the tumor. The resulting images were reviewed for quality and manually corrected when necessary.
Atlases.
Sex-specific spatial distribution atlases were generated for patient groups based on different categories, including overall population, MGMTp methylation, KPS, and extent of resection (EOR). Tumor epicenter and laterality were defined as the anatomic location of the primary lesion and the hemisphere with dominant tumor spread, respectively. Atlases were generated by using Python (Version 3.10.2) and Matlab (Version R2016b) by overlaying registered tumor segmentations for all patients in a group, calculating the frequency of tumor occurrence at each voxel and color-coding according to the frequency distribution.
Statistical Analysis
Missing values were noted in MGMTp methylation status, EOR, and preoperative KPS values (Fig 1). To address the missing data, data imputation was performed by using the Multiple Imputation by Chained Equations (MICE) package in R. MICE is a statistical tool that allows us to predict multiple missing variables from a data set by using regression modeling. To begin, imputation by using chained equations is performed when the data are “Missing At Random.” The program starts by performing imputation on each variable that is missing information and then regression analysis is performed to predict the missing value. This process is repeated in multiple cycles for each missing variable in the data set, thus producing a multiple imputed data set.22,23 Other variables included in our regression analysis are age, tumor volumes, epicenter, and laterality. Though alternative methods for handling missing data such as complete case studies exist, the accuracy of these methods to represent original data are significantly limited as compared with multiple imputations. Multiple imputations can handle data sets with up to 80% missing data and still produce unbiased results.24 Overall, we performed 10 imputations with 30 iterations. Prior literature suggests producing at least 5 imputation data sets with 5 iterations to reduce the Monte Carlo error.23 To impute the categoric variables, we used a polytomous (multinomial) regression model for all 3 variables (MGMTp methylation, KPS, and EOR).
To preserve the power of sample size and eliminate selection bias, sex-specific differences in tumor parameters were assessed by using nonparametric statistical tests in both the original data and the total data with imputation. The χ2 goodness-of-fit test was used to examine sex-specific proportional differences in categoric variables, whereas the Mann-Whitney U test was utilized for continuous variables. While performing statistical analysis on preoperative KPS scores, the patients are divided in to high-KPS (80–100) and low-KPS (below 80) categories. Previous literature has shown that patients with KPS of 80–100 are known to have better survival outcomes, thus these cutoff values are used in this study.25 Additionally, tumor volumes were analyzed before and after normalization to the total brain volume. False discovery rates (FDRs) were calculated by using the Benjamini-Hochberg method to control for type II error. Univariable and multivariable Cox proportional hazard analysis was conducted to calculate mortality ratios. Survival time differences among different categories were analyzed by using Kaplan-Meier curves and the log-rank test in the nonimputed data set. All statistical analyses were performed by using R (version 4.3.0). Graphs were generated by using Excel (Microsoft; version 2303) and R (version 4.3.0).
RESULTS
Overall Data: Original Data versus Total Data with Imputation
Men comprised 58.5% (n = 1066) and women comprised 41.4% (n = 754) of the total data set of 1820 patients. Women were diagnosed with glioblastoma at an older age (women: 64 years; men: 61.9 years; FDR = 0.003; Fig 2A). After imputation, a higher percentage of women had methylated MGMTp, though this difference was nearing significance in the original data. A higher percentage of men had a preoperative KPS value of 80 to 100 in both the original and imputed data sets. There were no sex-based differences with regards to the type of surgical procedure undertaken/EOR in either data set (Table).
Sex-specific differences in: (A) age at diagnosis, (B) enhancing (C), necrotic, (D) edema tumor volumes, and (E) tumor epicenter distribution of patients with glioblastoma. **P value and FDR < .05.
Voxel-based heat maps of tumor frequency in female and male patients with glioblastoma.
Descriptive statistics of sex-based tumor parameter distribution in patients with glioblastoma
Radiologic Parameters: Tumor Volumes and Epicenter
Women had smaller enhancing tumor volumes (median tumor volumes in cm3; women: 16.8; men: 20.8; FDR = 0.001), necrotic core volumes (median tumor volumes in cm3 women: 5.98; men: 7.87; FDR = 0.001), and edema volumes (median tumor volumes in cm3; women: 45.3; men: 58.9; FDR < 0.001) (Fig 2B). These differences were not significant after normalizing the tumor volumes to the whole brain volume.
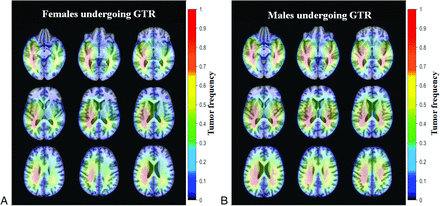
Overall, spatial maps depicting tumor laterality show higher frequency of right hemisphere tumors in women with a bihemispheric distribution pattern in men, though without statistical significance (Table, Fig 3). Regarding tumor epicenters, right temporal tumors were most frequently seen across the data set, however the overall distribution of tumor epicenters was distinct for men and women. In men, the most common epicenter regions were right temporal lobe, left temporal lobe, left frontal lobe, and right frontal lobe. In women, the most common epicenter regions were right temporal lobe, left frontal lobe, right frontal lobe, and left temporal lobe (Fig 2C). Overall temporal lobe involvement was more common in men. Interestingly, significant differences were found in the tumor laterality distribution based on MGMTp methylation, KPS, and EOR. Significantly higher percentages of bilateral tumors were seen in MGMTp-methylated women and men. Although not significant, a higher percentage of bilateral tumors were seen in MGMTp-unmethylated women and a higher percentage of right hemisphere and bilateral tumors were seen in men (Fig 4). Among men, 40.4% of those with a KPS below 80 had right hemisphere tumors. However, such patterns were not noted either in men with high KPS or in women, regardless of KPS. A significantly higher percentage of right hemisphere tumors were seen in women and men who underwent gross total resection (GTR) (Fig 5). Men who underwent sub-total resection (STR) and biopsy, and women undergoing biopsy had significantly higher frequency of bilateral tumors (Online Supplemental Data, Fig 6).
Voxel-based heat maps of tumor frequency in MGMTp unmethylated female and male patients with glioblastoma.
Voxel-based heat maps of tumor frequency in female and male patients with glioblastoma undergoing GTR.
Voxel-based heat maps of tumor frequency in female and male patients with glioblastoma undergoing STR.
Overall Survival
There were no sex-related disparities in the overall survival of patients with glioblastoma via a log-rank test (Fig 7A). The results of the multiparametric Cox-proportional hazard analysis of both imputed and original data revealed that patients with unmethylated MGMTp, higher age, and patients who underwent STR had a higher risk of mortality. Additionally, survival probability was significantly reduced in both patients with KPS < 80 in the original data and in patients with bilateral tumors and biopsy in the imputed data. There were no sex-based differences in mortality ratios within the total data with imputation and original data sets (Online Supplemental Data).
A, Sex-specific overall survival differences in patients with glioblastoma. B, Overall survival differences in US- and non-US-based population.
Sex-Specific Factors Influencing Survival
In the original data set, women with higher age, unmethylated MGMTp status, KPS < 80, and undergoing STR (instead of GTR) had increased risk of mortality. In men, higher age, unmethylation, and undergoing STR increased the mortality risk (Online Supplemental Data).
In the imputed data set, in women, higher age, MGMTp, unmethylated status, and undergoing STR or biopsy increased the mortality risk. In men, higher age, unmethylated MGMTp, undergoing STR or biopsy, and bilateral tumors (compared with tumors in left hemisphere) increased the risk of mortality (Online Supplemental Data).
US and Non-US Population
Patients based in the US (n = 1520) were diagnosed at a median age of 63 years as compared with 60 years in other regions of the world (n = 300; FDR < 0.001). A KPS of 80–100 was more frequent in the US-based population (KPS 80–100: US: 70.1%, non-US: 59.7%, FDR = 0.019). A significantly larger patient population from the US underwent GTRs, whereas, a higher percentage of STRs as compared with biopsy were undertaken by non-US based patients (EOR: GTR: US: 57.9%, non-US: 47.6%; STR: US: 32.9%, non-US: 44.9%; FDR < 0.001). A higher percentage of the non-US-based population had right hemisphere and bilateral tumors (laterality; right hemisphere: US: 33.2%, non-US: 40.4%; bilateral: US: 34.6%, non-US: 35.3%; FDR = 0.019). Larger tumor volumes were seen in enhancing tumor regions (median tumor volumes in cm3; US: 18.3; non-US: 24.9; FDR < 0.001), necrotic core regions (median tumor volumes in cm3; US: 6.6; non-US: 9; FDR < 0.001), and edema regions (median tumor volumes in cm3; US: 49.09; non-US: 68.4; FDR < 0.001) in the non-US data sets. There were no significant sex- and MGMTp-based differences in US and non-US regions (Online Supplemental Data). However, there was a median survival difference of 13 days between the non-US and US-based populations that was significant (median survival time in days: US population: 383; non-US population: 395.5; P = .0025; Fig 7B).
In the US data, a higher percentage of men had KPS of 80–100 (preoperative KPS 80–100; women: 64%; males: 75%; FDR = 0.083). Women were diagnosed at a median age of 64.8 years and men at 62 years (FDR = 0.001; Online Supplemental Data). These differences were not seen in the non-US patients. There were no other significant sex-based survival differences in these populations.
DISCUSSION
In this study, we analyzed sex-specific differences in tumor parameters and survival outcomes in patients with grade 4 IDH1-WT glioma by using the ReSPOND consortium data set. While there were several noteworthy differences in expression of various tumor parameters, sex-specific survival time differences were not seen.
MGMTp Methylation Status
Understanding sex-specific differences in various molecular parameters and their associations to survival outcomes is key for treatment planning. One such important molecular marker is the presence or absence of MGMTp methylation in grade 4 gliomas. MGMTp methylation enhances temozolomide treatment response and improves survival through various epigenetic mechanisms.26,27 Our findings show that MGMTp-unmethylated status increases the risk of mortality in men and women. In a previous paper looking at 3 distinct data sets, the authors found that, in addition to having a higher proportion of MGMTp methylation, women have a distinct survival advantage over men.4,27 These data sets were however considerably smaller than our sample size and at least 1 data set had a mix of IDH1-WT and mutant tumors included as glioblastoma.4,27
MGMTp-unmethylated patients also demonstrated interesting laterality trends as seen in Fig 4, with bihemispheric distribution in men and right hemispheric preponderance in women. Our results also replicate prior data to show a higher frequency of MGMTp methylation in female patients with glioblastoma4 compared with male patients in the imputed data set. While the exact reason for these sex-specific differences is unclear, notable sex-based differences are known to occur in the methylation patterns along CpG islands and regions near CpG islands (shores, shelves, and open seas) in patients with glioma.28 Based on these findings, MGMTp methylation is more frequent in women, possibly because a higher percent of methylations in women occur at the gene promoter regions.28
Tumor Volumes, Laterality, and Epicenter
There is limited literature on sex-based differences in radiologic parameters in patients with glioblastoma. Among these parameters, tumor volume has received the most attention; however, findings across studies have yielded inconsistent results. For instance, Bilello et al11 reported higher tumor volumes in female patients with grade 4 glioma, specifically in the necrotic core and enhancing tumor regions. In contrast, Colen et al12 found higher enhancing and necrotic tumor volumes in men, however, the observed sex-based differences in enhancing tumor volumes did not reach statistical significance. We observed overall significantly lower tumor volumes in the necrotic core, edema, and enhancing tumor regions among women. For the sake of completeness, we presented results with and without normalization to whole brain volume. However, considering the sexual polymorphism observed in brain volumes and cortical thickening across various brain regions, we believe normalization poses a risk of masking true differences.29
Sex-specific methods for tumor volume normalization is a well-debated topic in the scientific literature.30 A study examining 5216 adult brains from the UK Biobank demonstrated higher brain volume in 11 regions (such as the left isthmus cingulate) and 13 regions (such as the right superior parietal) in men and women, respectively. Higher cortical thickening was observed in 25 regions (such as the right insula) and 24 regions (such as the left inferior parietal) in men and women, respectively.29 Additionally, either using total brain volume or intracranial brain volume for normalization has been shown to mask crucial sex-based differences in brain volumes. For example, thalamic volumes were found to be higher in men, however, after brain volume normalization this difference becomes statistically insignificant. This study has also shown greater sex-related influence on brain volumes before normalization.31 Most studies looking at sex-specific differences in tumor volumes have not performed normalizations.11,12 Therefore, we presented the data before and after tumor volume normalization to total brain volume.
Molecular mechanisms may aid in elucidating the underlying reasons for sex-based differences in tumor volumes, particularly within the necrotic volume. Notably, the aforementioned study by Colen et al12 identified associations between apoptotic TP53 and oncogenic MYC pathways with cell death in men and women, respectively. Furthermore, they also noted reduced expression of p53 protein in female patients.12 Because the MYC pathway induces cell death through the ARF-MDM2-p53 pathway, the increased expression of the MYC gene along with reduced expression of p53 in female patients with grade 4 glioma suggests diminished cell death.32 These findings offer a plausible explanation for the sex-based differences observed in necrosis volume in our study.
Previous investigations examining sex-based differences in tumor epicenter and laterality have revealed contrasting findings.11,33,34 Specifically, one study reported a greater involvement of the right temporal region in women and left temporal lobe in men.11 Another study showed no significant sex-based differences in tumor epicenter distribution.34 Our results showed an overall higher frequency of frontotemporal tumors, with both women and men exhibiting a greater percentage of right temporal tumors. However, the overall involvement of temporal lobes was higher in men.
Tumor volume and epicenter are two important factors that determine the clinical presentation and type of interventions including the extent of resection and radiation therapy volumes. In our study, larger tumor volumes in men did not result in significantly worse KPS scores or higher frequency of biopsies or partial/subtotal resections. A study by Hadi et al35 has shown that larger tumor volumes increase the risk of radiation necrosis in patients with glioma undergoing stereotactic brachytherapy. A future direction of research could investigate sex-specific differences in overall rates of radiation necrosis and their relationship to tumor volumes.
Survival Outcomes and Sex-Specific Factors Influencing Survival
Studies investigating sex-based differences in survival have yielded conflicting results.2,4,5,36⇓-38 For example, Gittleman et al4 examined the US-based National Cancer Database and observed a 3-month survival difference favoring women, while studies conducted on non-US populations have generally not identified significant sex-based variations in survival outcomes.5,9,36,38 Notably, these studies include all grade 4 gliomas in their analysis.
Our data set did not show significant sex-specific differences in overall survival, despite a larger proportion of women having methylated MGMTp. The lack of survival advantage in women can probably be attributed to lower preoperative KPS values in this data set. Additionally, the prognostic factors affecting mortality ratios in men and women were not significantly distinct. Several of the known mortality predictors were identified (age, unmethylated MGMTp, EOR) in line with previous literature,2,4,7,37 whereas preoperative tumor volumes were not a predictor of mortality. This is not entirely inexplicable as a recent study by the Response Assessment in Neuro-Oncology group showed that in comparison with preoperative tumor volume, the EOR and residual contrast-enhancing tumor volume are better predictors of survival in IDH-WT glioblastomas.39
Geographic Location of the Patient
Previous literature and our study have shown association between geographic location of the patient and their tumor parameters and survival outcomes.15,16 The overall tumor volumes were larger in US patients as compared with non-US populations. However, US patients also had a higher rate of GTR. US patients had slightly lower overall survival, which could be correlated to their higher age at diagnosis and the lower percentage of patients undergoing STR as compared with biopsy. Conversely, non-US patients had a higher frequency of undergoing STRs as compared with GTRs and biopsy. Multiple factors can potentially contribute to geographic differences such as racial distribution, ethnicities, education, insurance, and treatment availability.40 More studies are necessary to define these associations.
LIMITATIONS
This study is subject to potential selection bias due to variations in inclusion and exclusion criteria across institutions. Our results are based on pretreatment data, and the potential impact of posttreatment changes on radiologic and clinical parameters are not accounted for.41,42 The US-based population in our cohort is significantly larger than the non-US population, limiting the inference of our geographic analysis. Binary categorization of patient population into US- and non-US-based is not ideal, because heterogeneity in the non-US regions is still not accounted for. The data set has a limited non-US cohort (n = 300 versus US = 1540) limiting the analysis to binary category. Missing data on certain tumor parameters were a limitation. However, we addressed the missing data with multiple imputations.
CONCLUSIONS
This is a comprehensive clinico-radio-genomic analysis of sex-based differences in a large IDH1-WT grade 4 glioma multinational cohort. We found that glioblastomas demonstrated notable sex-based differences in various tumor parameters, including MGMTp methylation status, KPS score, tumor epicenter, and tumor volumes. There were no significant sex-specific disparities in survival times, mortality ratios, or mortality predictors.
Footnotes
↵# Christos Davatzikos and Chaitra Badve contributed equally to this article.
This research has been supported by National Institutes of Health/National Cancer Institute (1RO1CA269948-01); Imaging Devices and AI Technologies Track Funding Agency: Jobs Ohio.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received February 14, 2024.
- Accepted after revision April 17, 2024.
- © 2024 by American Journal of Neuroradiology