Abstract
SUMMARY: We report a patient with neurocysticercosis who developed numerous cerebral edematous lesions while undergoing cysticidal therapy. These lesions outnumbered viable cystic lesions seen before therapy. Most new lesions were subsequently found to be associated with former calcifications not seen on initial MR imaging. Calcified neurocysticercosis lesions can trigger inflammatory reactions during therapy, and the number and location of calcified neurocysticercosis lesions may influence treatment decisions.
Neurocysticercosis is among the most prevalent inflammatory central nervous system diseases in most developing countries. Because of migration and global traveling, neurocysticercosis is also increasingly observed in nonendemic areas.1 Disease symptoms are usually caused by inflammatory reactions associated with the dying process of parasites.2,3 Although these lesions show gadolinium enhancement on MR imaging, nonenhancing viable cystic lesions are often asymptomatic.4 Calcifications, the final stage of neurocysticercosis, are often asymptomatic but have recently been found to be the cause of epilepsy in a substantial number of patients.5 Intermittent inflammatory changes in calcified lesions, sometimes symptomatic, have also been described.6 We present a case with inflammatory reaction in numerous calcified lesions at the time of cysticidal therapy.
Case Report
A 43-year-old Vietnamese woman who had immigrated to Germany 7 years previously presented with recurrent episodes of tingling of the right forearm.
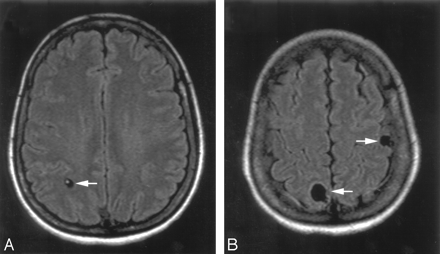
Electroencephalography showed focal right slowing without epileptic discharges. Because a peripheral lesion responsible for the recurrent paresthesias was ruled out by neurography, these events were interpreted as simple partial seizures. Neurocysticercosis was diagnosed serologically and by MR imaging, which showed 10 cystic lesions and 1 enhancing cerebral lesion. Three cystic lesions are shown in Fig 1. Numerous calcifications were found on CT of the brain. MR imaging was performed at 1.5T. Three months before treatment, fluid-attenuated inversion recovery (FLAIR) (retention time [TR]/echo time [TE], 6114/150) and T1-weighted images (TR/TE, 600/13) with and without contrast were obtained. After treatment, FLAIR (TR/TE, 7500/110) and T1-weighted imaging (TR/TE, 525/17), again with and without contrast, were performed; section thickness was 5 mm for all images.
Fluid-attenuated inversion recovery (FLAIR) imaging studies before therapy. Three of 11 cystic lesions are shown: living parasite in a right parietal cystic lesion (white arrow, A); another right parietal and a left parietal cortical cystic lesion (white arrows, B).
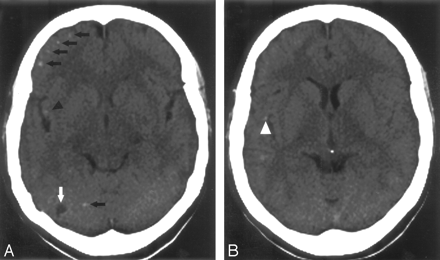
The patient was treated with a combination of albendazole, dexamethasone, and clobazam for 8 days.7 Ten days after completing this regimen, the patient presented again with a severe holocephalic headache and fever. Repeat MR imaging showed 25 inflammatory lesions with ring enhancement. Edematous changes on FLAIR images were seen in 15 of these locations. All lesions seen on the initial MR imaging showed this pattern (compare Fig 2A–C and D–F for a right frontal lesion as an example). Three “new” lesions represented inflammation of previously unrecognized small cysts. However, 8 enhancing lesions were localized to previous calcifications on CT, and 3 were located at sites of initially normal-appearing parenchyma. Two of the new lesions corresponding to calcifications in the right perisylvian area are shown in Figs 2D–F and 3. Three months after therapy, MR imaging showed only 3 slightly edematous lesions, 2 of which were associated with former cysts (1 is shown in Fig 2I), and one with previously normal-appearing brain parenchyma. The patient is currently asymptomatic.
Treatment response of the patient shown by MR imaging. Imaging was performed before treatment (A–C) and 10 days (D–F) and 3 months (G–I) after therapy. A–C, A small cystic right frontal lesion without contrast enhancement (A, B) or edema (white arrow, C) is seen; in the right perisylvian area, hypointense lesions medially (open triangle) and laterally to the right sylvian fissure are seen on fluid-attenuated inversion recovery (FLAIR) but not on T1-weighted images. D–F, Soon after therapy, ring enhancement (D, E) and edema (F) are detected in the right frontal lesion (white arrow); ring enhancement with severe edema is seen in the perisylvian lesions (open and white triangles; E, F). G–I, Three months after treatment, a slight enhancement (G, H) and edema (I) are still present frontally (white arrow); no lesion is seen in the perisylvian area (open and white triangles; H, I). A, D, G are T1-weighted images without contrast; B, E, H, T1-weighted images with contrast; and C, F, I, FLAIR images.
CT scans obtained 3 months before therapy. The calcifications medially (triangle, A) and laterally (white triangle, B) to the right perisylvian fissure correspond to the perisylvian lesions shown in Fig 2 D–F. Note several calcifications not showing an inflammatory reaction after therapy (black arrows, A) and an additional right cerebellar lesion (white arrow, A).
Discussion
Active immune evasion by a living parasite has been suggested to explain why viable cystic neurocysticercosis lesions usually do not cause inflammation and are commonly asymptomatic.8,9 Parasitic death leads to a breakdown of this mechanism, leading to an inflammatory reaction, which can cause an encephalitic syndrome in many patients, with temporary clinical deterioration. This can be provoked by cysticidal therapy.1 The condition of our patient, not surprisingly, deteriorated while she was undergoing cysticidal therapy. This patient presented with lesions attributable to different stages of neurocysticercosis, which are seen only in a minority of patients and may represent reinfection.1
On MR imaging rescanning after therapy, we found evidence of inflammatory lesions, not only at locations corresponding to cystic lesions before therapy but also in other locations. Although some of the additional lesions corresponded to small cystic lesions that had not been noticed before, 8 lesions localized to calcifications seen on the pretreatment CT scan and 3 to initially normal-appearing brain tissue. Inflammatory reactions in these locations are most likely immunologically driven. Although antigen liberation occurs during the process of parasitic dying,1 these antigens can also persist in calcified lesions. During and after cysticidal therapy of numerous viable lesions, high quantities of antigen are released by the simultaneous death of parasites, resulting in a strong immunologic activation.10 As shown in our report, calcified remnants of parasites can be targeted by this process, probably involving parasite-specific T cells and macrophages. Subsequent to degradation of the cysts, calcifications can only be detected after a latent period. The length of this period has not yet been defined exactly, but it is thought to last 1 to several years.4 The inflammatory reactions at previously normal-appearing locations in our patient might correspond to such lesions.
Only recently, specific guidelines for cysticercosis therapy have been released.11 Therein, recommendations for patients with different numbers of cysts or calcifications have been given. No consensus has been reached for patients with very few (< 5) or extremely high numbers (>100) of viable lesions. However, most experts agree that a higher burden (5–100) of viable cysts should be treated with concomitant steroids during antihelminthic therapy. In patients with exclusively calcified lesions, neither steroids nor antiparasitic treatment should be administered because such lesions represent already dead cysticerci.11 The situation of large numbers of calcifications in combination with a relatively small number of cystic lesions, as in our patient, has not however been specifically addressed. Calcified lesions may lead to inflammation during and after cysticidal therapy, which might cause substantial clinical problems after antihelminthic therapy in patients with unfavorably localized calcifications (eg, close to the mesencephalic duct).
Conclusion
We propose that the number and localization of calcifications in patients with few viable cysts should influence therapeutic decisions. Concomitant administration of steroids during cysticidal therapy in patients with many or unfavorably localized calcifications in addition to viable cysts might be beneficial.
Acknowledgments
We thank Xiomara Pedré-Villareal for expert assistance with the figure preparation.
References
- Received March 31, 2005.
- Accepted after revision May 9, 2005.
- Copyright © American Society of Neuroradiology