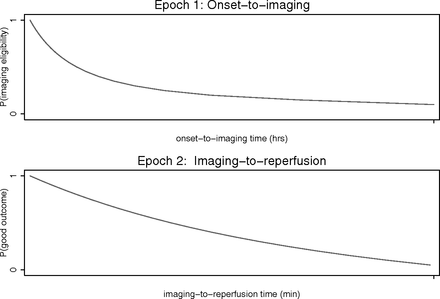
There are 2 “epochs” of time in acute ischemic stroke caused by large vessel occlusion: onset to imaging time, which is deterministic of the likelihood of favorable imaging (mild to moderate early ischemic changes [ASPECTS 6–10]), and imaging to reperfusion time, which is deterministic of the likelihood of a favorable outcome.1 But what factors influence whether a particular patient with an acute stroke caused by large vessel occlusion will have favorable imaging? What is the rate at which the brain dies after stroke onset? What factors influence the velocity of irreversible infarction?
Ten minutes after stroke onset caused by large vessel occlusion, all patients will have a small core and sizeable penumbra. At the other extreme, in nearly all patients at 24 hours after stroke onset, the infarct will have expanded to its maximum volume and there is no penumbra. The decay curve for growth of infarct (expansion of core, reduction of penumbra) for an individual patient begins at 100% salvageable brain (zero core) at onset and follows a variable downward curve to reach 0% salvageable brain at a certain point (Figure).2,3
Interval times in acute stroke (modified from Hill et al17). With increasing data, we have a good understanding of the second curve (imaging to reperfusion). However, our understanding of the first curve remains limited because of a paucity of appropriate data.
Some patients are likely “fast progressors” (with favorable imaging only very early) and others are “slow progressors” (with favorable imaging even at late time windows). The biologic infarct growth curve could also be linear, parabolic (steep initially and flattening out as time progresses), or even sigmoid shaped (slow infarct growth initially that increases as time progresses, then flattens out at later time points).3,4 Recent analyses of workflow time relationships for both intravenous tPA and endovascular treatment attest to the variable nature of the infarct growth outcome relationships.4⇓⇓⇓–8 In particular, in a recent meta-analysis of all the endovascular trials, a nonlinear statistical exploration of the time-versus-outcome relationship showed a shallow slope very early, with a steep fall in good outcome rate from 190–390 minutes after stroke onset and a gradual decline later (see Fig 5 in Saver et al4). The nature of this time-versus-outcome relationship may likely be very different if patients with large infarcts at baseline (fast progressors) or those with minimal clinical deficits (very slow progressors) who were likely excluded from the recent intra-arterial therapy trials that were included in this analysis. Although “time is brain” is an established construct, we currently have very limited data on the time-versus-outcome relationship in all comers and how this relationship may be different in different groups of patients.
We also have little quality data on why some patients are fast progressors and some are slow progressors. All the recent trials (overtly or inadvertently) used imaging or clinical parameters that resulted in the inclusion of patients with a small core independent of time from onset.9 So, by definition, nearly all patients in the later time windows had to be slow progressors. Fast progressors were excluded from these trials. A strong candidate as a pathophysiologic variable to explain the differences between slow and fast progressors is the status of leptomeningeal collaterals. The better the collaterals, the slower the progression of infarct. So what influences the presence of good collaterals, and what do we understand about it? It is likely that collaterals are influenced by genetic factors and coexisting conditions such as diabetes and hypertension. A second candidate variable is tissue susceptibility, which, to date, is impossible to measure in isolation and is poorly defined and understood. Another variable that likely comes into play in patient selection is tissue eloquence (nearly all patients who were enrolled in the recent trials had clinically major stroke symptoms; hence, it is possible that there are patients who have sizeable noneloquent tissue at risk, but were not included in the trials because of clinically mild symptoms).
Animal data suggest that there are significant genetic influences on the robustness of collaterals.10 Other risk factors associated with poor collaterals include aging, hypertension, diabetes, or the presence of metabolic syndrome and hyperuricemia.11 The use of statins and angiotensin converting–enzyme inhibitors may be associated with good collaterals.12 Furthermore, the immediate physiology of collaterals may be acutely influenced by systemic blood pressure, locoregional factors such as carotid artery stenosis, or the degree of vessel occlusion caused by a large bore catheter and other modifiable factors.
Tissue susceptibility may be modifiable. Multiple compounds have been shown to be cytoprotective in ischemia-reperfusion models in rodents and other preclinical models. None have been proved in human stroke. Variables that are explanatory for tissue susceptibility include age and sex, premorbid brain health (perhaps measured crudely by functional status), comorbid conditions (such as diabetes mellitus, congestive heart failure, and cancer). Among these patients, the impact of a moderate stroke may be much worse. Both novel and well-known compounds such as NA-1,13 minocycline,14 and uric acid15 may change tissue susceptibility, perhaps to a varying degree depending upon the patient.
So where do we go from here? The shape of the infarct growth curve is unknown. Variable rates of infarct growth likely exist because of variability in robustness of collaterals and tissue susceptibility to ischemia. We have limited understanding of the factors that influence these variables. Data to understand this issue in humans are limited by sampling biases stemming from current patient selection strategies in available studies. As a first step, we need better databases comprising all patients with acute ischemic stroke caused by large vessel occlusion. Such prospective databases should capture clinical information that includes time of onset, age, comorbidities, and information regarding vital signs (eg, “the patient was not hypotensive”). Imaging data should include modalities that assess the presence of a sizable penumbra indirectly “collateral information” or directly “perfusion imaging.” Finally, laboratory investigations should be added for conditions that are known to impact the penumbra/collateral status, such as blood glucose, uric acid, or metabolic syndrome work-up. Clinical follow-up and outcome data irrespective of how patients were treated are essential. Such data bases could be an expansion of existing stroke registries (eg, Austrian Stroke Unit Registry Collaboration16) or previous randomized trials that captured data on the patients who were screened for eligibility, but excluded because of unfavorable imaging. These will help advance our understanding of which factors are associated with the fast progressor state and, hopefully, could be modified to improve the stroke outcome of these patients.
Footnotes
Disclosures: Mayank Goyal—UNRELATED: Research Grants: Medtronic, Comments: partial funding of ESCAPE trial*; Consultancy: Medtronic, Stryker, Microvention, Comments: teaching and advice on acute stroke techniques and devices; Grants/Grants Pending: Medtronic, Comments: HERMES collaboration*; Patents (Planned, Pending, or Issued): GE Healthcare, Comments: licensing agreement for development of systems of stroke diagnosis. Andrew Demchuk—UNRELATED: Payment for Lectures (Including Service on Speakers Bureaus): Medtronic. Michael Hill—UNRELATED: Board Membership: Canadian Neuroscience Federation, Comments: not-for-profit board; Consultancy: Merck, Comments: adjudication panel for clinical trials outcomes panel (paid work); Grants/Grants Pending: Boehringer Ingelheim, Medtronic, Comments: grant to the University of Calgary for the TEMPO-two trial (Boehringer Ingelheim) and for the ESCAPE trial and the HERMES collaboration (Medtronic)*; Stock/Stock Options: Calgary Scientific Inc, Comments: private company stock ownership. *Money paid to the institution.
References
- © 2017 by American Journal of Neuroradiology