Abstract
Sarcoidosis is a multisystem granulomatous disease, with intramedullary spinal cord involvement seen in <1% of cases. This case series illustrates the clinical presentations and imaging findings of 5 patients with intramedullary spinal neurosarcoidosis occurring at sites of spondylotic spinal canal stenosis, which can be indistinguishable from spondylotic myelopathy with cord enhancement. Both entities are most common in middle-aged men and present with weeks to months of motor and sensory symptoms. On imaging, both can have focal spinal cord enhancement and longitudinally extensive signal abnormality centered at or just below the level of spinal canal stenosis. On the basis of our experience, we suggest that in patients with cord enhancement centered at or just below a site of spinal canal stenosis, consideration should be given to chest imaging and lymph node biopsy when applicable, to assess for the possibility of underlying sarcoidosis before surgical decompression.
ABBREVIATIONS:
- ACE
- angiotensin converting enzyme
- WBC
- white blood cell
Sarcoidosis is a chronic multisystem granulomatous disease characterized by the accumulation of noncaseating epithelioid granulomas.1 Sarcoidosis involving the spine is rare and may include extradural, intradural, or intramedullary lesions, with intramedullary spinal cord involvement seen in <1% of cases.2,3 However, when spinal cord involvement is part of the disease course, it is frequently the first manifestation of the disease.1,4 These patients will often have asymptomatic disease elsewhere, such as hilar adenopathy.1 Patients with intramedullary spinal neurosarcoidosis typically present with progressive paresthesia, proprioceptive disturbances, weakness, and/or sphincter dysfunction, with symptoms often occurring for months before the diagnosis is made.1
Spondylotic myelopathy is a more common cause of spinal cord dysfunction. When involving the cervical spine, the term “spondylotic myelopathy” has recently been replaced with “degenerative cervical myelopathy,” which is now the recognized terminology in practice guidelines by AO Spine (https://www.aofoundation.org/spine) and has been accepted by multistakeholder evaluation.5 Nevertheless, the term “spondylotic myelopathy” has persisted throughout the literature, and we will use the term spondylotic myelopathy in this article for consistency and because some of our cases involve the thoracic spine. There are reports in the literature of a subset of cases of spondylotic myelopathy in which prominent spinal cord edema and focal enhancement of the cord are seen.6 The mechanism for the edema and enhancement has been speculated to be disturbed venous circulation caused by spinal cord compression resulting in venous hypertension at the affected level and a breakdown of the blood-cord barrier.7 Most of these patients initially present with motor weakness and sensory disturbance, gait disturbance, and exaggerated deep tendon reflexes, with symptoms usually present for several months before diagnosis.6
Intramedullary spinal neurosarcoidosis occurring in association with spondylotic spinal stenosis has not, to our knowledge, been specifically described in the radiology literature, with a few cases previously described in the neurosurgery and neurology literature.8-10 In some reported cases of presumed spondylotic myelopathy, the intramedullary abnormality of neurosarcoidosis was initially incorrectly ascribed to spondylosis, leading to ineffective decompressive surgery and subsequent progression of neurosarcoidosis owing to lack of appropriate treatment.
We report a series of 5 patients who presented to our institution from 2018 to 2019 and were diagnosed with intramedullary spinal neurosarcoidosis at the level of mass effect on the spinal cord due to either a disc herniation or spondylotic spinal canal stenosis. Four of these patients did not have a previous diagnosis of sarcoidosis, with cord involvement being the initial symptomatic lesion. Most interesting, the imaging appearance of our patients is strikingly similar to that in reported cases of spondylotic myelopathy. Spondylotic myelopathy was initially considered as a diagnostic possibility for most of our patients before we arrived at the diagnosis of sarcoidosis based on an intrathoracic lymph node biopsy.
This retrospective study was approved by our institutional review board.
CASE SERIES
Case 1
A 31-year-old man presented with 6 months of progressively worsening bilateral arm and leg weakness and numbness. On examination, his strength was 4/5 proximally and 3/5 distally in both his arms and legs. He had absent sensation to light touch and temperature in the bilateral upper extremities. Reflexes were 2+ in the upper extremities and 4+ in the lower extremities.
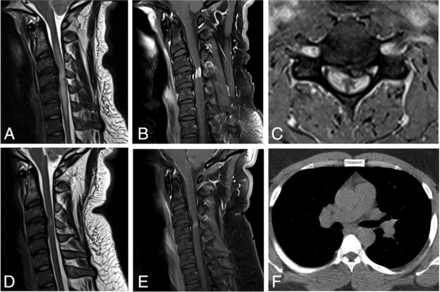
CSF analysis showed a white blood cell (WBC) count of 24/μL with lymphocytic pleocytosis, an elevated protein level of 54 mg/dL (reference range, 15–45 mg/dL) and an elevated glucose level of 114 mg/dL (reference range, 40–70 mg/dL). The serum angiotensin converting enzyme (ACE) level was normal at 53 U/L (reference range, 9–67 U/L). MR imaging of the cervical spine showed a prominent disc osteophyte complex at C3–4. There was long-segment cord edema extending from C2 through C7, with focal enhancement just inferior to the C3–4 intervertebral disc (Fig 1A–C). Findings were thought to be most consistent with spondylotic myelopathy. Due to the cord enhancement, a chest CT was obtained to assess for sarcoidosis. The chest CT showed micronodularity in a perilymphatic distribution and hilar adenopathy, favoring sarcoidosis (Fig 1F). A transbronchial lymph node biopsy was performed, showing lymphocytes and non-necrotizing granulomas.
A 31-year-old man with 6 months of progressively worsening bilateral arm and leg weakness and numbness. Sagittal T2-weighted (A) image shows a prominent disc osteophyte complex at C3–4 with spinal canal stenosis and long-segment cord signal abnormality. There is focal cord enhancement centered just below the level of compression on postcontrast sagittal (B) and axial T1-weighted (C) sequences, with involvement of the peripheral cord and relative sparing of the central GM. There is persistent-but-decreased cord signal abnormality and enhancement at the 23-month follow-up on sagittal T2-weighted (D) and postcontrast T1-weighted (E) sequences. Noncontrast chest CT at presentation (F) shows bilateral hilar adenopathy.
A steroid pulse was started after the biopsy, and the patient had rapid improvement in symptoms. He was then treated with prednisone, 60 mg daily, with a prolonged taper and adalimumab; he was later transitioned to infliximab. At imaging follow-up, the cord signal abnormality and enhancement improved but persisted at last follow-up at 23 months (Fig 1D, -E).
Case 2
A 52-year-old woman presented with 5 months of gradually worsening numbness from the waist down, weakness of the right hand and lower extremities, and urinary incontinence. She had a known history of pulmonary sarcoidosis. On examination, right upper-extremity strength was 3/5; left upper-extremity, 4/5; right lower-extremity, 1/5; and left lower-extremity, 3/5. The Hoffman sign was positive.
CSF analysis showed 7 WBC/μL, a mildly elevated protein level of 54 mg/dL, and normal glucose levels. The serum ACE level was normal (11 U/L). MR imaging of the cervical spine showed spondylotic changes with maximal canal stenosis at C6–7. There was spinal cord signal abnormality spanning C4 through T4, with associated enhancement at C6 and C7 (Online Supplemental Data). Diagnostic possibilities offered included neurosarcoidosis versus spondylotic myelopathy. Chest CT showed very mild hilar adenopathy, related to known sarcoidosis.
She was started on IV methylprednisolone sodium succinate, and the paresthesia and lower-extremity weakness significantly improved the following day. She was then treated with a combination of infliximab and prednisone. At 13-month imaging follow-up, there was improved-but-persistent cord signal abnormality and enhancement (Online Supplemental Data).
Case 3
A 51-year-old man presented with 4 months of progressively worsening subjective, bilateral, lower-extremity weakness and bowel/bladder dysfunction. On examination, strength was 5/5 in the upper and lower extremities. Sensation to light touch was intact in all 4 extremities, and gait and reflexes were normal.
CSF analysis showed a WBC count of 237/μL, with lymphocytic pleocytosis. There was mild elevation of the CSF protein level at 59 mg/dL. Glucose level was normal. The serum ACE level was within normal limits at 54 U/L. MR imaging of the cervical and thoracic spine was performed and showed a disc protrusion at T7–8. There was cord signal abnormality spanning T4 to T11 and enhancement at T7 and T8 (Online Supplemental Data). Differential considerations offered included demyelinating disease, sarcoidosis, and spondylotic myelopathy. A chest CT showed mild mediastinal and bilateral hilar adenopathy. An endobronchial biopsy of the lymph nodes showed non-necrotizing granulomas in a background of lymphocytes.
He was treated with a 5-day course of IV methylprednisolone with improvement in symptoms, then maintained on prednisone and methotrexate. Five-month imaging follow-up showed marked improvement in abnormal enhancement and expansion of the thoracic cord (Online Supplemental Data).
Case 4
A 35-year-old man presented with 1 month of progressively worsening lower-extremity weakness, paresthesia, and difficulty with ambulation. On examination, he had 3/5 strength in the bilateral lower extremities.
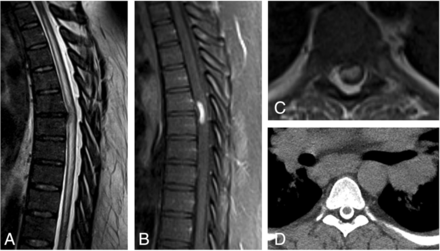
CSF analysis showed 32 WBC/μL with lymphocytic pleocytosis, a mildly elevated protein level at 51 mg/dL, and mildly elevated glucose level at 88 mg/dL. Serum ACE and CSF ACE levels were within normal limits at 63 and 1.8 U/L (reference range, 0.0–2.5 U/L), respectively. Thoracic spine MR imaging showed a right central disc extrusion at T6–7. There was cord signal abnormality from T3 to T11 and focal ventral cord enhancement at T6–7 (Fig 2A–C). The imaging differential diagnosis included neoplasm or sarcoidosis because hilar adenopathy was also noted on the MR imaging. Hilar and Mediastinal adenopathy were confirmed on a CT myelogram (Fig 2D). Endobronchial lymph node biopsy showed lymphocytes and granulomatous inflammation.
A 35-year-old man with 1 month of progressively worsening lower-extremity weakness, paresthesia, and difficulty with ambulation. Sagittal T2-weighted (A) image shows a disc extrusion at T6–7, with long-segment cord signal abnormality centered at this level. Postcontrast sagittal (B) and axial T1-weighted (C) sequences demonstrate focal cord enhancement at the level of the disc extrusion. CT myelogram (D) performed for further evaluation of the cord abnormality shows marked bilateral hilar adenopathy.
He was started on IV dexamethasone with improvement in symptoms. MR imaging of the thoracic spine performed after 4 days on steroid therapy showed a decrease in enhancement and edema. He was started on 30 mg of prednisone daily and has not received long-term follow-up at our institution.
Case 5
A 51-year-old man presented with 1–2 weeks of bilateral foot numbness that progressed up his legs, as well as urinary incontinence and saddle anesthesia. He was evaluated at an outside hospital where MR imaging showed cervical spondylosis, worst at C5–6 and C6–7, with disc osteophyte complexes causing moderate spinal canal stenosis at these levels. There was cord signal abnormality extending from C3 to T5, centered around enhancement in the dorsal and peripheral cord at C7 and T1 (Online Supplemental Data). A chest CT showed mediastinal and hilar adenopathy, and a biopsy was performed, with lymph nodes showing granulomatous inflammation. He was given dexamethasone with slight improvement in the numbness and urinary symptoms. Following a 2-week course of steroids, he presented to our institution with worsening lower-extremity symptoms. On examination, he had decreased pinprick and temperature sensations over the back of his thighs and in his feet. Strength was normal in all 4 extremities. Reflexes were 2+ and symmetric.
No report of CSF analysis was available from the initial work-up. Following the initial course of therapy, a CSF examination at our institution demonstrated a mildly elevated glucose level at 72 mg/dL but was otherwise unremarkable. MR imaging of the cervical and thoracic spine showed improved-but-persistent cord signal abnormality and enhancement. Internal interpretation of the outside lymph node biopsy showed lymph nodes with numerous non-necrotizing granulomas, thought to be consistent with sarcoidosis.
He was treated with prednisone. At 4-month follow-up, the patient’s symptoms of paresthesia in the lower extremities and right buttocks were unchanged. His examination findings had improved, with improved temperature sensation in the lower legs. Follow-up MR imaging at 5 months showed decreased-but-persistent enhancement in the cord (Online Supplemental Data).
DISCUSSION
This case series illustrates the clinical presentations and imaging findings of isolated intramedullary spinal neurosarcoidosis occurring at sites of spondylotic spinal canal stenosis. This entity can be indistinguishable both clinically and on imaging from spondylotic myelopathy. Both are most common in middle-aged men and present with weeks to months of motor and sensory symptoms. On imaging of both entities, there can be focal spinal cord enhancement and longitudinally extensive signal abnormality centered at or just below the level of spinal canal stenosis. We will also discuss a possible explanation for the coincidence of intramedullary spinal neurosarcoidosis occurring at the site of maximal spinal canal stenosis.
Intramedullary Spinal Neurosarcoidosis
Sarcoidosis is a systemic inflammatory disorder characterized by the development of noncaseating granulomas. The pathophysiology of sarcoidosis remains elusive. Granuloma formation is initiated by T lymphocytes responding to a specific-but-currently unknown antigen.11 The most common sites of involvement are the lungs and lymph nodes; however, any part of the body can be affected. Although rare, when the spine is involved, it is most often in the form of neurosarcoidosis, with intramedullary and/or leptomeningeal involvement. Sarcoidosis can also involve the extradural spine, including the vertebrae or rarely the epidural space or discs.11,12 While the appearance is highly variable, the classic enhancement pattern of neurosarcoidosis in the cord is of dorsal-subpial enhancement, which, when present, in combination with central canal enhancement, can resemble a trident head on axial images.10,13 There is often more longitudinally extensive cord signal abnormality on the T2-weighted images,4 as seen in all our cases. The appearance is not specific for the disease, so in patients with intramedullary spinal neurosarcoidosis without systemic involvement, the correct diagnosis is often difficult and delayed.3 Correlation between symptom resolution and resolution of imaging findings in neurosarcoidosis is poor, especially with spinal cord lesions. Enhancement typically decreases with treatment but may persist for years. In our series, long-term follow-up scans revealed persistent, small amounts of enhancement in 3 of 4 cases.
For intramedullary spinal neurosarcoidosis, steroids can result in remarkable recovery, particularly if treatment is started early in the course of the disease.3 However, the disease may only partially resolve and may recur, especially if there is a delay in diagnosis and treatment.3,14 It is recommended that corticosteroid therapy be started as early as possible, before irreversible gliofibrosis occurs in the cord. Conditions of some patients may continue to deteriorate despite treatment, and they can end up with paraparesis. Therefore, patients with spinal cord sarcoidosis typically are committed to a long duration of therapy with gradual tapering of steroids,14 and they often require additional immunosuppressant therapy.15
In many cases of intramedullary spinal neurosarcoidosis, biopsy is not desirable due to the morbidities associated with biopsy of the spinal cord. A diagnosis of probable neurosarcoidosis can be based on clinical or imaging evidence of lesions, with evidence of systemic sarcoidosis obtained from a biopsy of another organ. Four of our patients had lymph node biopsies revealing granulomatous inflammation, and the fifth patient had a known history of pulmonary sarcoidosis. The diagnosis can be supported by ≥2 other findings, such as typical chest adenopathy or elevated serum ACE levels,11 though ACE levels are only elevated in 5%–50% of cases. PET/CT can be more sensitive to systemic involvement and could be considered if chest CT findings are equivocal or negative,16 particularly if there is high clinical suspicion of sarcoidosis based on other factors such as family history or genetic predisposition. The CSF examination most often shows nonspecific elevation in protein levels and lymphocytosis. All our patients who had CSF laboratory values available before initiating therapy had elevated lymphocyte levels and mild elevation in protein levels. Two of our patients also had elevation of glucose levels.
Spondylotic Myelopathy
There are a number of case reports and publications in the literature that describe a pattern of spondylotic myelopathy with cord enhancement and T2 signal abnormality. One of the first case reports was in 2008 by Cabraja et al.17 They noted a case of a 36-year-old man with 3 months of slowly progressive symptoms who was found to have contrast enhancement localized directly at the site of greatest narrowing of the spinal canal.
In 2010, Kelley et al18 published a case series of 5 cases of suspected spondylotic myelopathy with cord edema and focal enhancement at the site of maximal stenosis. After decompression, none had recurrent myelopathy with a mean follow-up of 27 months.
In a case series by Nurboja et al19 in 2012 of 6 patients with cervical spondylotic myelopathy, 1 patient failed to improve following decompressive surgery and was later found to have neurosarcoidosis on the basis of chest x-ray, serum ACE levels, and chest mediastinoscopy. They suggested an algorithm for treating these patients, starting with a referral to a neurologist with further possible investigations including MR imaging of the brain, CSF analysis, hematologic and biochemical analysis, and nerve-conduction studies, to exclude other causes such as inflammatory, demyelinating diseases or neoplasms. If no neurologic cause is found, then spinal cord decompression should be considered. After satisfactory surgical decompression, regular follow-up should be arranged with serial MR imaging, with biopsy if there is further clinical or radiologic deterioration.19
In 2013, Flanagan et al20 introduced a distinctive type of enhancement that can occur in spondylotic myelopathy, termed “pancake-like enhancement,” with the term referring to enhancement that is wider than tall when viewed in the sagittal plane. Flanagan et al21 later assigned 3 guidelines for the enhancement to be considered pancake-like: 1) a transverse band appearance, greater in transverse than in vertical extent on sagittal images, 2) location just below the site of maximal stenosis at the center of a spindle-shaped T2 hyperintensity, and 3) circumferential enhancement sparing GM on axial images. In this retrospective analysis, the characteristic pancake-like enhancement was seen in 41/56 patients (73%) with cord enhancement associated with spondylotic myelopathy.21 After the operation, 39 patients reported some improvement, 14 reported stable symptoms, and conditions of 3 continued to deteriorate despite the operation. Enhancement was found to persist in the cord in 100% at 3 months, 75% at 12 months, and 62% at last follow-up. Hilar adenopathy was seen in 2 of 33 chest CTs, and 3 patients had pathologic evidence of systemic sarcoidosis. Marked clinical and radiologic improvement after an operation without immunosuppressant treatment excluded neurosarcoidosis as the cause of the myelopathy in 1 of the 3 patients. In the other 2 patients, sarcoidosis may have contributed to the myelopathy, but spondylosis was considered more likely due to the pancake-like enhancement and improvement after the operation. They noted that while this pancake-like enhancement pattern is strongly indicative of spondylotic myelopathy, the exclusion of alternative causes of myelopathy associated with gadolinium enhancement is impossible with complete certainty.21
Intramedullary Spinal Neurosarcoidosis at the Level of Cervical Spondylosis
Regarding intramedullary spinal neurosarcoidosis occurring as isolated CNS involvement only at sites of spinal canal stenosis, it has long been postulated that other inflammatory lesions, specifically MS plaques, tend to occur at sites of cord trauma and degenerative stenosis, with close anatomic correspondence between mechanical stresses on the cord by spondylosis and spinal cord MS plaques.22,23
The trauma could result in loss of blood-cord barrier impermeability, which could allow the entrance of the antigen responsible for sarcoidosis into the cord as well as the entrance of T lymphocytes to set off the inflammatory cascade. As stated by Hickey,24 “any mechanism that physically destroys the components of the blood cord barrier will render the CNS open to the cellular and molecular constituents of the blood. The participants for inflammation can then be rapidly delivered to the site of injury in a gross, nonspecific fashion.” This explanation would fit very well with the pattern that we observed in our 5 cases, with no disease elsewhere along the neuroaxis aside from that centered at or just below the site of maximal spinal canal stenosis.
In 2011, Sakai et al9 reported a retrospective review of 12 patients with spinal cord sarcoidosis and concomitant cervical spondylosis. They noted the possibility that compressive cervical myelopathy could trigger the development of inflammatory granulomas in spinal cord sarcoidosis and that the MRIs showed that areas of enhancing sarcoid lesions coincided with the maximum compression levels. One-third of the patients in their study had temporary improvement in symptoms after decompressive surgery. They concluded that in spinal cord sarcoidosis accompanied by compressive myelopathy, decompressive surgery should not be the first choice of treatment. They also noted that the outcome was poorer in patients with neurosarcoidosis and compressive myelopathy compared with those without compressive myelopathy.9 More recently in a study of phenotypes of sarcoidosis-associated myelopathy, Murphy et al10 described a pattern of anterior thoracic myelitis associated with areas of disc degeneration and suggested that there may be a predilection for involvement of areas of the spinal cord susceptible to mechanical stress. They also suggested that in some cases, sarcoidosis and spondylosis may both be contributing to the clinical and imaging picture.
The differential diagnosis for intramedullary spinal cord enhancement with associated longitudinally extensive signal abnormality in the cord is broad and includes sarcoidosis, spondylotic myelopathy, neuromyelitis optica, spinal cord tumor, and dural arteriovenous fistula. Spondylosis is a common incidental finding in patients presenting with cord abnormalities. When the spinal cord enhancement and edema are centered at or just below the site of maximal spinal canal stenosis, the primary considerations should be intramedullary spinal neurosarcoidosis and spondylotic myelopathy. While our cases fulfilled some of the rules set forth by Flanagan at al,21 including location just below the site of maximal stenosis in the center of a spindle-shaped T2 hyperintensity and circumferential enhancement sparing GM on axial images, none of our cases had a band of enhancement that was greater in the transverse than in the vertical extent on sagittal images. This feature may be the most useful finding to differentiate spondylotic myelopathy from intramedullary spinal neurosarcoidosis.
CONCLUSIONS
Intramedullary spinal neurosarcoidosis can have an identical imaging appearance and clinical presentation to spondylotic myelopathy, and intramedullary spinal neurosarcoidosis can be isolated only to sites of spondylotic spinal canal stenosis. We believe that in patients with cord enhancement centered at or just below a site of spinal canal stenosis, consideration should be given to chest imaging and lymph node biopsy if there is nodal enlargement. These recommendations are in addition to those previously put forth by others. A presurgical course of corticosteroids should be considered to see if there is improvement or resolution of symptoms without surgery. While urgent surgery may be warranted if a patient presents acutely, many of these patients present with subacute-to-chronic symptoms, allowing time for a trial of corticosteroids. It is also possible that some patients may ultimately benefit from surgical decompression in addition to medical management of sarcoidosis due to the coexistence of the 2 pathologies.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
- 8.
- 9.
- 10.
- 11.
- 12.
- 13.
- 14.
- 15.
- 16.
- 17.
- 18.
- 19.
- 20.
- 21.
- 22.
- 23.
- 24.
- Received September 12, 2022.
- Accepted after revision October 21, 2022.
- © 2023 by American Journal of Neuroradiology