Abstract
BACKGROUND AND PURPOSE: We report our experience with MR imaging, MR angiography, and catheter angiography in children with acute idiopathic cerebral infarction and suggest that catheter angiography may still play an important role in this setting.
METHODS: During the past 8 years, 18 children with idiopathic cerebral infarction underwent MR imaging and catheter angiography; 17 were also studied with MR angiography. MR imaging was done within 34 hours after onset of hemiplegia or seizures or both. Sixteen patients underwent catheter angiography within 36 hours of MR imaging; 12 studies were performed within 22 hours. Two patients underwent catheter angiography, in both cases within 72 hours. Infarcts were compared with arterial abnormalities seen at catheter angiography, and the results of MR angiography were compared with those seen at catheter angiography.
RESULTS: Comparing MR angiography with catheter angiography, we found the positive predictive value of MR angiography for arteriopathy was 100%, with a negative predictive value of 88%. MR angiography was equivalent to catheter angiography in the detection and depiction of proximal middle cerebral artery disease; however, depiction of disease in the internal carotid artery (ICA) and detection of peripheral embolic disease were better with catheter angiography than MR angiography.
CONCLUSION: Basal ganglia lesions associated with ICA disease by MR angiography should probably be studied with digital subtraction angiography, as MR angiography did not depict the length and severity of ICA disease as well as catheter angiography did. Hemispheric infarcts should be studied with catheter angiography, as emboli may occur in the absence of heart disease; the circle of Willis may be uninvolved with embolic disease, and MR angiography is not sensitive to emboli in small peripheral intracranial arteries.
Ischemic cerebral infarction is rare in childhood; unpublished statistics suggest a regional prevalence of 1.7 per 100,000 population in the Dallas-Fort Worth area, and other studies have reported the frequency, excluding children with sickle cell disease, to be less than 1 in 100,000 (1). The risk factors for childhood stroke include relatively common disorders, such as congenital heart disease, trauma, infection, and sickle cell anemia, as well as uncommon metabolic disorders, such as homocystinuria (1, 2). In 25% to 50% of affected children, no risk factors can be found (1, 2). No formal recommendations have been published for the radiologic evaluation of acute childhood stroke. Along with MR imaging, MR angiography is often performed in this setting. Although there are anecdotal reports on the use of MR angiography in childhood infarction, the literature has not established the efficacy of MR angiography in pediatric stroke. Catheter angiography increases the cost of evaluation, especially if general anesthesia is required, and adds some potential patient risk, although in the hands of neuroradiologists experienced in pediatric angiography, the risk is minimal.
In this article, we review our 8-year experience with the use of MR imaging, MR angiography, and catheter angiography in the evaluation of childhood idiopathic cerebral infarction to compare the location of the infarct with arterial abnormalities seen at catheter angiography and to compare the findings at MR angiography with the abnormalities seen at catheter angiography.
Methods
Between January 1991 and January 1999, 60 children with acute nonhemorrhagic cerebral infarction were seen at our institution. Routine laboratory tests in this clinical setting include complete blood cell count and platelet count, erythrocyte sedimentation rate, prothrombin time/partial thromboplastin time, lupus anticoagulant, serum amino acids, antithrombin III, protein C and S, anticardiolipin antibody, triglycerides, cholesterol, lactate, pyruvate, and sickle cell preparation, when appropriate. Excluding neonates and children with risk factors identifiable by clinical history, physical examination, CSF analysis, or routine laboratory tests, 19 children had no identifiable risk factors. Eighteen of these had been examined with MR imaging and catheter angiography; the 19th patient had MR imaging and MR angiography but did not undergo catheter angiography. The 18 patients who underwent MR imaging and catheter angiography constitute the study population and ranged in age from 4 months to 16 years (mean age, 7 years). There was no sex predominance. Presenting symptoms were hemiparesis in 15, four of whom also had seizures, and seizures without fixed neurologic deficits in three, one of whom also had choreiform movements.
All patients underwent MR imaging within 34 hours of symptom onset, although no one was studied within the first 6 hours. MR imaging was done with a 0.5-T superconducting unit in seven patients and with a 1.5-T unit in 11. Three patients had been studied at an outside institution with a 1.5-T unit. Until 1996, the MR imaging examinations at our institution included sagittal and axial T1-weighted spin-echo (SE) sequences and proton density–and heavily T2-weighted dual-echo conventional SE sequences. Since 1996, T2 information has been acquired with a fluid-attenuated inversion-recovery sequence (TR/TE = 8000/120; TI = 2300) and a T2-weighted fast SE sequence (TR/TE = 3000/120; TSE = 13). Twelve of 18 patients had contrast-enhanced T1-weighted imaging. MR angiography was done in 17 of the 18 patients and included the entire cranial vault and the skull base. The imaging parameters for the 3D time-of-flight (TOF) MR angiographic sequences were 35/4.9 (TR/TE), 19° flip angle, 1-mm slice thickness, and 200-mm field of view. Two patients had intracranial 3D phase-contrast angiography (velocity-encoding value = 30–70 cm/s) and seven patients had 2D TOF MR angiography of the cervical carotid arteries. The source images from the MR angiographic sequences were reconstructed using a maximum intensity projection (MIP) algorithm. Faculty pediatric neuroradiologists prospectively interpreted the MR images and MR angiograms, in all cases in advance of conventional angiography.
Sixteen patients had catheter angiography at our institution within 36 hours of MR imaging; in 12 cases, within 22 hours. Two patients had catheter angiography at an outside institution; in these cases, the exact time that elapsed between MR imaging and catheter angiography is not known, although both were studied within 72 hours. At our institution, angiography was performed under general anesthesia or deep sedation. Angiography included an arch aortogram in four patients, although aortography is not usually performed or recommended in this clinical setting. Sixteen patients had angiography of the common carotid arteries. Selective bilateral internal carotid and vertebral arterial injections were performed using standard angiographic projections as well as additional projections to visualize the middle cerebral artery (MCA) bifurcations. In one case, angiography preceded digital subtraction angiography (DSA) and was done using cut-film. The two patients in whom angiography was performed at an outside institution did not undergo angiography of the common carotid arteries; one patient had been studied using cut-film angiography. A faculty interventional neuroradiologist reviewed all catheter angiograms, blinded to the results of MR imaging and MR angiography. The prospective interpretations of the MR angiograms were compared with findings at DSA for the internal carotid arteries (ICAs) and the proximal MCAs ipsilateral to the cerebral infarction as well for the ICA and MCA supplying the hemisphere contralateral to the infarct, and the results were entered into a database.
Statistical analysis was performed using Fisher's exact test combining data comparing MR angiography and DSA of the ICA and proximal MCA from the unaffected and affected hemispheres. Phi coefficients were also determined as an additional measure of association.
Results
MR Imaging
MR images showed isolated basal ganglia infarcts in 12 patients, including one in whom the infarction was limited to the posterior limb of the internal capsule (Table 1). Involvement of the basal ganglia was limited to the putamen in seven of the 13 patients with basal ganglia infarcts. Four of the 12 patients with a basal ganglia infarct had abnormal T2 signal in the adjacent centrum semiovale. One patient had basal ganglia infarct and involvement of the ipsilateral insular and parietal cortex. Two patients had bihemispheric cortical infarcts, and one had infarction limited to the left posterior parietal region. One patient had unilateral anterior and posterior watershed infarcts. In one patient, MR imaging showed no evidence of infarction at presentation or on a repeat examination 5 days later, although there was thrombus within the distal ICA contralateral to the hemiparesis. The infarcts had no side predominance.
Clinical and neuroradiologic attributes in 18 children with idiopathic ischemic cerebral infarction
MR Angiography
Seventeen patients underwent MR angiography. These findings were prospectively interpreted as normal in four patients, three with basal ganglia infarcts and one with a left parietal infarction (Table 1). In six patients, MR angiograms were interpreted as showing unilateral stenotic or occlusive disease limited to the proximal MCA ipsilateral to the infarction. Seven patients had stenotic or occlusive disease of the ICA shown by MR angiography. The two patients with bihemispheric cortical infarcts had bilateral ICA disease; one of these had moyamoya disease revealed by SE images. One patient had bilateral ICA disease associated with watershed infarction limited to one hemisphere. The interpreting neuroradiologist considered the MR angiogram to be of poor quality in seven of 17 patients. Four had steno-occlusive disease of the carotids and were imaged at 0.5 T, while one had proximal MCA stenosis and was imaged at an outside institution, which included MR imaging at 1.5-T and 3D phase-contrast MR angiography.
Angiography
The arch aortograms obtained in four patients and the common carotid angiograms obtained in 16 of 16 patients were normal. At presentation, cranial DSA findings were normal in three patients, showed angiographic abnormalities limited to the proximal MCA in six patients, and disclosed uni- or bilateral carotid disease in seven patients. One patient had occlusion of the carotid artery associated with subtle stenosis or spasm of the ipsilateral MCA; one patient had unilateral multiple vessel involvement, and one had unilateral embolic disease to the left posterior parietal artery (Table 2). The patient with angiographic evidence of distal emboli had no heart disease and the source of the emboli remains unknown. In one of the three patients with normal findings at angiography, stenosis of the MCA developed ipsilateral to the infarct, and was seen at DSA 3 months later; one of the patients with MCA stenosis progressed to unilateral moyamoya disease 18 months after initial presentation. Three patients with isolated proximal MCA disease underwent repeat DSA 6 months later; the arterial disease was unchanged.
Comparison of site of infarct with arteriopathy at digital subtraction angiography
Of the seven patients with carotid disease, two had cervical carotid dissections and the other five had involvement of the distal carotids. The supraclinoid involvement varied from complete occlusion in the setting of moyamoya disease to subtle narrowing thought to be due to spasm.
Comparison of MR Imaging Abnormalities with Findings at Catheter Angiography
Basal ganglia infarction was associated with stenosis or occlusion limited to the proximal MCA in six patients (Fig 1), disease of the ICA in three patients (Fig 2), no angiographic abnormalities in three patients, and definite angiographic abnormalities of more than one vessel in one patient (Table 2). Hemispheric involvement, not seen in any patient with isolated angiographic abnormalities of the proximal MCA, was associated with steno-occlusive disease of the ICA in three patients (Fig 3) and with embolic disease in one patient. In one patient who had both basal ganglia and ipsilateral hemispheric infarction, DSA showed multiple arterial stenoses of the circle of Willis. The patient with hemiparesis, who had no MR imaging evidence of infarction, had occlusion of the ICA contralateral to the hemiplegia (Fig 4) as well as subtle narrowing of the ipsilateral MCA. In this patient, spasm of the MCA could not be distinguished from stenosis, and follow-up angiography was not performed.
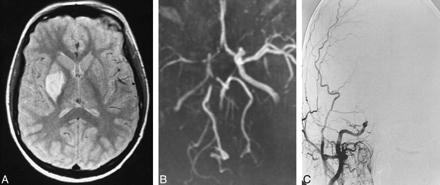
Patient 8: 4-year-old boy with right hemiparesis. This case illustrates the typical location of MCA stenosis associated with basal ganglia infarction.
A, Axial proton density–weighted image shows infarct in the left putamen.
B, Coronal MIP from 3D TOF MR angiography (0.5 T) shows stenoses (arrows) at the M1-M2 junction.
C, Stenoses of the left MCA (arrows) correspond in location to those seen at MR angiography. The angiographically more severe lesion is associated with a longer area of dephasing on the MR angiogram. There was no change in the arterial disease at repeat DSA 6 months later.
Patient 6: 13-year-old girl with carotid occlusion associated with basal ganglia infarction. This case illustrates the insensitivity of MR angiography to distal carotid occlusion when flow through the circle of Willis is intact. By MR angiography, the right ICA was patent and the MCA was small.
A, Proton density–weighted image shows infarct of the right putamen.
B, Axial MIP from 3D TOF MR angiography (0.5 T) shows small-caliber right MCA and A1 segments, suggesting disease in both vessels. The right ICA appears patent but was actually occluded at DSA.
C, DSA shows abrupt occlusion of a small-caliber ICA at the level of the ophthalmic artery. The MCA and A1 segments were normal with injection of the left ICA.
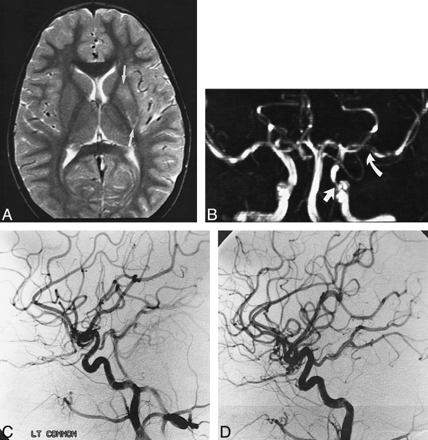
Patient 4: 15-year-old girl with left hemiparesis. In this case, carotid occlusive disease could not be distinguished from stenosis, and the extent and severity of occlusive disease were overestimated by MR angiography.
A, Axial proton density–weighted image shows watershed infarcts.
B, Coronal MIP from MR angiography acquired at 0.5 T provides poor delineation of the circle of Willis and ICA.
C, Anteroposterior projection shows supraclinoid ICA is of smaller caliber than the A1 and M1 segments, indicating stenosis. The left ICA was occluded.
Patient 10: 6-year-old boy with right hemiparesis that resolved over 6 days. In this case, ICA dissection was associated with questionable narrowing of the MCA.
A, Coronal MIP from 3D TOF MR angiography shows small-caliber low signal intensity in the left ICA (arrow), indicating slow flow.
B, Left common carotid angiogram shows dissection of the left cervical carotid artery, which terminates at the level of the ophthalmic artery.
C, Right ICA angiogram shows subtle stenosis or spasm of the left MCA (arrow), which was not perceived at MR angiography. Follow-up angiography was not performed.
Comparison of MR Angiography and DSA
With DSA as the standard of reference, MR angiography was judged to be inferior to DSA in seven of the 17 patients who underwent both MR angiography and DSA, usually because of inadequate lesion characterization. In one patient, the ICA stenosis appeared considerably more severe by MR angiography than at catheter angiography, and there was artifactual narrowing on the coronal MIP, not seen on the axial source images or the multiplanar reformatted images (Fig 5). As illustrated in Figure 2, ICA occlusion was consistently indistinguishable from stenosis by MR angiography. MR angiography, however, compared favorably with DSA for lesion detection. MR angiographic findings were true-positive for detection of arterial disease in 17 of 34 hemispheres examined, false-negative in two, false-positive in none, and true-negative in 15. The positive predictive value of MR angiography for arteriopathy was 100%, and the negative predictive value was 88%. Analysis by a two-tailed Fisher's exact test revealed P < .0005, indicating a significant association between MR angiography and DSA for detection of carotid or MCA disease. Another measure of correlation, phi value = .89, also supported good correlation between MR angiography and DSA.
Patient 13: 5-year-old boy with right hemiparesis. This case illustrates overestimation of ICA stenosis by MR angiography as compared with DSA.
A, T2-weighted MR image shows infarct in left putamen (arrows).
B, Coronal MIP from 3D TOF MR angiography shows narrowed left ICA (straight arrow). Apparent narrowing of left MCA (curved arrow) is a reconstruction artifact that was not present on the axial source or multiplanar reformatted images.
C, Selective left ICA injection done 17 hours after MR angiography shows apparent concentric narrowing (possibly spasm) of the supraclinoid ICA. The MCA was normal.
D, Lateral view of the right ICA is shown for comparison. Note the caliber of the supraclinoid ICA.
Thirteen (76%) of 17 patients with MR imaging evidence of infarction had lesions in the basal ganglia. Twelve of these 13 patients had both MR angiography and catheter angiography. Among these patients, MR angiography was true-positive for detection of steno-occlusive disease of the ICA or proximal MCA in 10 hemispheres examined, false-positive in none, true-negative in 13, and false-negative in one. Compared with DSA, the positive predictive value of MR angiography for proximal MCA disease in this group of patients was 100%, with a negative predictive value of 93% (P < .0005, phi = .92).
Discussion
The literature contains several reports of pediatric stroke series, although most predate MR imaging, lack correlation between MR imaging and catheter angiography, and provide no recommendations for optimizing imaging strategies in this clinical setting (3–6). As in adults, diagnostic imaging should be directed toward rapid identification of arterial disease that is potentially amenable to endovascular or medical intervention. Despite the lack of published reports documenting the efficacy of MR angiography in childhood stroke, in our institution, MR angiography is usually requested instead of catheter angiography.
The distribution of infarction among our patient population is similar to that reported in the literature, in that 72% had involvement of the basal ganglia (4–6). The extent and location of the infarction within the basal ganglia as reported in the literature are variable. Brower et al (7) described 36 children under 14 years of age with basal ganglia or thalamic infarctions associated with a diverse group of pathogeneses and risk factors. Twenty-nine children had involvement of the globus pallidus or putamen or both, and 23 had infarction involving the caudate. In our study of children in whom no risk factors were known, the putamen was preferentially involved.
The most common clinical presentation of basal ganglia infarct is hemiplegia, which occurs in more than 90% of children (7). Altered mental status, sensory deficits, aphasia, or ataxia are unusual with basal ganglia infarcts, suggesting that the ischemic insult is limited to the basal ganglia or that the hemispheric ischemia is clinically occult. Residual neurologic impairment tends to be considerably less than that seen in adults with radiologically comparable strokes (6). In our study, 10 (77%) of 13 patients with basal ganglia infarcts were left with minor motor deficits.
With the exception of children with congenital heart disease, in whom strokes tend to be embolic in origin, the most common site of arterial disease depicted at angiography is the distal ICA or proximal MCA (3). In 1971, Harwood-Nash et al (3) described the findings in a large series of children with acute hemiplegia who were studied with cerebral angiography. Steno-occlusive disease was seen in the supraclinoid ICA in 24 of the 40 patients and in the MCA in 20 patients (3). Within the MCA, involvement of the proximal MCA was twice as frequent as was involvement of the distal MCA. This large study preceded the advent of CT, and no correlation between angiographic abnormalities and site of infarction was possible. In a smaller study, 29 children with hemiplegia and no known risk factors were analyzed (8). By angiography, 24 of these patients had carotid disease, with a preponderance of lesions in the carotid siphon and supraclinoid regions; the MCA was involved in only four patients.
The pathogenesis for carotid occlusive disease is speculative, although there have been reports of inflammatory arteritis, cystic medial necrosis, and spontaneous dissection (1, 3, 9). Several authors have commented on the frequency with which upper respiratory infections precede acute hemiplegia (3, 4, 10); however, these reports are anecdotal and may be coincidental given the high frequency of upper respiratory infections in children.
A correlation between CT and angiographic abnormalities in pediatric basal ganglia infarction was reported by Zimmerman et al (11), who described 15 children with basal ganglia infarcts studied with CT. Eleven of the 15 underwent arteriography, which was positive in 10 cases. Excluding the three patients with known lipoprotein abnormalities or sickle cell anemia, eight children had no identifiable risk factors. Angiography showed stenosis of the MCA without involvement of the anterior cerebral artery (ACA) in two patients, intracranial ICA dissections in two, extracranial ICA dissection in one, and one each had embolic disease, moyamoya disease, and aneurysm.
As has been reported in the literature and as we observed in our study, basal ganglia infarction may occur without angiographic abnormalities. Explanations for this phenomenon include angiographically occult intrinsic arteriopathy limited to the lenticulostriates, disruption of the lenticulostriates as a result of unrecognized head trauma, and thromboembolic disease of the carotid arteries with rapid dissolution of the saddle emboli. It has been stated that basal ganglia infarcts in children are due to occlusive disease of the lenticulostriates at their origin, as compared with adults, in whom there is occlusion of the distal lenticulostriates (11). The scientific basis for this statement, however, has not been established.
In our series, hemispheric infarction was seen in four patients and was associated with carotid disease in three. Carotid disease was more often associated with basal ganglia infarction than with hemispheric infarction. The frequency of hemispheric infarction in children with ICA occlusion is lower than that observed in adults. Harrison and Marshall (12) reviewed CT studies of 61 adults with angiographically confirmed ICA occlusion and found that 20 had no CT evidence of stroke. Infarcts were small and cortical in 11, whereas 12 had multiple infarcts within a vascular territory supplied by the ICA. Ten patients had large confluent infarcts in the vascular distribution of the MCA: one of these had both MCA and ACA infarcts, one had infarcts in the MCA and the posterior cerebral artery (PCA), one had PCA infarcts only, and four had white matter lesions, three of which were classic watershed infarcts. Involvement of the internal capsule was noted in three patients and of the globus pallidus in one patient (12).
Mechanisms of cerebral ischemia in adults with severe stenotic or occlusive disease of the carotid artery include vascular occlusion from an embolism or thrombus propagating from an atherosclerotic plaque and watershed infarction caused by inadequate perfusion pressure (13). Leptomeningeal collateral flow may explain basal ganglia infarction with hemispheric sparing in carotid occlusive disease. Children presumably have a pial arteriocapillary bed that dilates, compensating for diminished perfusion by reducing vascular resistance at a microcirculation level; oligemic parenchyma, occult by conventional MR imaging techniques, may have been present (13). We did not measure regional cerebral blood volume or any other parameters of cerebral perfusion or oxygen extraction, as these techniques have only recently been available. Studies of regional cerebral blood flow and cerebral blood volume would have been particularly illuminating in the one patient in our study with carotid occlusion who presented with hemiplegia and had no MR imaging abnormalities.
Identification of arterial disease is important for patient management. At our institution, patients with idiopathic cerebral infarction associated with angiographic abnormalities are anticoagulated for 3 to 6 months and placed on antiplatelet agents. Rapid, accurate, noninvasive depiction of arterial disease is clearly preferable to invasive angiography. The use of MR angiography in children with suspected intracranial vascular abnormalities was reported in 1992 by Vogl et al (14), who studied 24 children with MR angiography. Of the eight patients in whom arterial stenoses were identified at MR angiography, only four had DSA. DSA confirmed the MR angiographic findings in three of these four patients. The conclusion was reached that MR angiography “compared favorably” with DSA (14). In our study, MR angiography was comparable to DSA in the detection of stenotic or occlusive disease of the ICA and MCA but not in the detection of embolic disease. The insensitivity of MR angiography to small vessel disease is a known problem. MR angiography was also inferior to catheter angiography in depicting the length and severity of some arterial lesions, mostly in the ICA. The accurate representation of ICA disease is important, as some patients may potentially be candidates for thrombolytic therapy, angioplasty, or, conceivably, carotid stent placement. The mid-field MR imaging unit used to study some of the patients did not have sufficient gradient strength for the short TEs required for optimal MR angiography. Severely stenotic lesions could not be differentiated from frank occlusion. Even with high-field MR imaging, however, overestimation of the severity and length of a stenosis by MR angiography as compared with DSA is a known pitfall (15).
A comparison of the length and severity of an arterial lesion seen by MR angiography with that seen at DSA may be excessively rigorous and ignores the physiologic information about blood flow provided by MR angiography. As this study predated the availability of diffusion-weighted and perfusion imaging and MR spectroscopy at our institution, this retrospective evaluation focused on the presence and extent of vessel occlusion. We undertook this study to determine whether MR angiography provides sufficient anatomic information such that DSA is not necessary for patient management. Some inherent bias was introduced into the study, as the neuroradiologists were not double-blinded to the results of MR imaging, MR angiography, and catheter angiography. Larger series of patients with acute hemiplegia of childhood must be studied prospectively using standardized pulse sequences on high-quality MR imaging units, and the results must be statistically analyzed before recommendations can be issued that endorse the use of MR angiography over DSA in this clinical setting. Given the rarity of childhood stroke, accumulation of statistically significant numbers of affected patients would require an interinstitutional collaborative effort with standardized MR imaging protocols.
Conclusions
MR imaging and MR angiography should be obtained from all children with acute hemiplegia, as well as diffusion-weighted imaging, perfusion imaging, and MR spectroscopy, where possible. Classic basal ganglia infarction associated with a normal MR angiogram or with an MR angiogram showing a normal ICA and disease of the proximal MCA probably does not require DSA. Given the good correlation between MR angiography and DSA for detection of MCA lesions and the unsuitability of these lesions for angioplasty, patient management decisions will not be substantially altered by the addition of DSA. Because catheter angiography is superior to MR angiography in the characterization of ICA abnormalities, catheter angiography should be considered when basal ganglia infarcts are associated with carotid disease as depicted by MR angiography or when hemispheric infarction is present. When cortical infarcts are seen at MR imaging, with or without basal ganglia infarcts, DSA is indicated, as MR angiography is not sensitive to small vessel disorders associated with embolic disease.
Acknowledgments
We acknowledge Linda Hynan for statistical assistance and Allison Russell for photography.
Footnotes
References
- Received June 18, 1999.
- Copyright © American Society of Neuroradiology