Abstract
BACKGROUND AND PURPOSE: Perfusion MR imaging, performed as dynamic-susceptibility contrast-enhanced MR imaging, is sensitive to hemodynamic risks for patients with cerebrovascular disease. We sought to define a quantitative parameter for perfusion MR imaging, which shows brain areas at hemodynamic risk and enables direct comparison of different perfusion MR imaging examinations.
METHODS: A new standardization procedure for the time-to-peak (TTP) parameter, standardized time to peak (stdTTP), was introduced. The stdTTP automatically calculates a time offset correlated to the earliest enhancing voxels in a section and rescales all TTP values accordingly. Because of a close relation between this offset and stdTTP of early enhancing voxels in central vascular territories (CVTs), stdTTP provides an estimate of the bolus run time between CVTs and related border zones (BZs). The stdTTP in CVTs and BZs was measured in 11 patients without hemodynamic impairment by using high temporal resolution dynamic-susceptibility contrast-enhanced perfusion MR imaging.
RESULTS: An excellent comparability of different dynamic susceptibility contrast-enhanced MR imaging studies was found. The stdTTP in CVTs was 0.4 ± 0.5 s (minimum, 0 s; maximum, 1.3 s) for the anterior, 0.5 ± 0.3 s (minimum, 0 s; maximum, 1.0 s) for the middle, and 1.4 ± 0.5 s (minimum, 0.4 s; maximum, 2.4 s) for the posterior cerebral artery. In the anterior BZ, stdTTP was 2.3 ± 0.4 s (minimum, 1.6 s; maximum, 3.2 s), and in the posterior BZ, stdTTP was 2.8 ± 0.4 s (minimum, 2.0 s; maximum, 3.4 s).
CONCLUSION: The results suggest a limit for stdTTP of approximately 3.5 s in the anterior and posterior BZs. The stdTTP could serve as a quantitative measure for the hemodynamic risk assessment of patients with cerebrovascular disease. Because stdTTP can be directly derived from the measured curves, the hemodynamic situation of a patient can be judged with a minimum of computational effort.
Perfusion MR imaging performed as dynamic-susceptibility contrast-enhanced MR imaging (DSC MR imaging) is sensitive for judgment of the hemodynamic risk of patients with cerebrovascular disease. Mean transit time and time to peak (TTP), both time parameters available with DSC MR imaging, were reported to be sensitive for detection of hemodyamic alterations (1, 2). Although mean transit time is of higher computational expense, TTP can be directly and quantitatively derived from the measured curve. Unfortunately, direct comparisons of TTP between different patients or follow-up examinations of the same patient are difficult to obtain because of different bolus travel times of the contrast medium. Although this problem can be solved by using an arterial input curve (3, 4), this method needs higher demands on the technical capabilities of the MR imager and the consecutively necessary calculations. Therefore, a simple automatic offset calculation procedure for standardization of TTP (stdTTP), which needs no special input curve, was introduced, and the practicability of this new parameter was tested in this study.
Methods
Eleven patients (seven women and four men; age range, 20–41 years) underwent examinations because of epileptic foci screening. They had no history of cerebrovascular disease. Stenotic alterations of cerebral vessels were excluded with sonographic examinations and MR angiography (3D phase-contrast technique; velocity, 1.1 m/s) of cervical and cerebral vessels. All patients had morphologically normal MR images.
DSC MR imaging was performed on a 1.5-T imager (Gyroscan ACS-NT; Philips, Best, The Netherlands) using a dynamic T2*-weighted echo-planar fast-field-echo MR sequence (74/30/1 [TR/TE/excitations]; flip angle, 30°), with a 240-mm field of view and an acquisition matrix of 128 × 128 voxels. The images were reconstructed within a matrix of 256 × 256 voxels. Two sections with 7-mm thickness and a 0.7-mm gap were measured 164 times per minute. The temporal resolution was therefore approximately 355 milliseconds. Imaging was performed in the axial plane at the level of the basal ganglia. A contrast medium bolus (dose, 0.15 mmol/kg) was administered through a 20-gauge venous cannula in a cubital vein by using an MR motor injector (Spectris; Medrad-Europe, The Netherlands) with an 8 mL/s flow rate. An injection delay, relative to the start of the dynamic sequence, of 15 seconds was introduced to get a sufficient number of baseline images. After DSC MR imaging, T1-weighted spin-echo MR imaging (500/15/2) was performed to exclude blood-brain–barrier disruption.
Calculations
The data from the DSC MR images were transferred to a personal computer workstation (Intel-PII processor), equipped with a software package that was developed in our department (program language, C++). After automatic mask leveling, which was performed to restrict the calculations to voxels in brain parenchyma only, the dynamic curves were analyzed and primary TTP maps drawn. The point of time when the first 3% of the evaluated voxels had reached their peak enhancement was calculated as the intrinsic offset for each section, and all TTP values were then rescaled relative to this offset. Therefore, the TTP values were interpreted afterward as stdTTP (5). 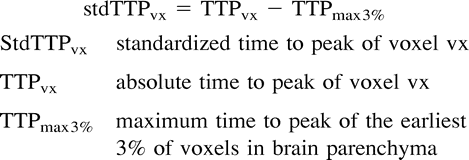 where stdTTPvx is the stdTTP of voxel vx, TTPvx is the absolute TTP of voxel vx, and TTPmax3% is the maximum TTP of the earliest 3% of voxels.
where stdTTPvx is the stdTTP of voxel vx, TTPvx is the absolute TTP of voxel vx, and TTPmax3% is the maximum TTP of the earliest 3% of voxels.
Afterward, in all patients, the mean stdTTP of regions of interest covering the earliest enhancing voxels of the vascular territories of the anterior, middle, and posterior cerebral arteries was measured. These voxels should represent the stdTTP of the central vascular territories (CVTs). In the same way, regions of interest covering the latest enhancing voxels in a vascular territory were introduced and their mean stdTTP measured. These voxels should be representative for the border zones (BZs). The average size of the regions of interest was 1.81 cm3. Because stdTTP provides an absolute time measure (unit, s), rational scaling of the data was assumed. Descriptive statistics (mean and SD) were obtained, and, for comparisons between groups, analysis of variance (post hoc procedure, Bonferoni) was conducted.
Results
None of the patients showed any sign of blood-brain–barrier disruption, which might have influenced the calculations. In all patients, the CVT of the anterior, middle, and posterior cerebral arteries and the anterior and posterior BZs could be identified in both hemispheres by using stdTTP.
In total, 110 regions of interest (44 BZs, 66 CVTs) were evaluated. No significant difference between the left and the right side was found for the CVTs or BZs (analysis of variance: groups = 10, n = 110, P > .05 [not significant]). Therefore, the data from corresponding CVTs and BZs from both sides were grouped and the mean and SD calculated (Table). A reference value, calculated as mean + (2 · SD), of 3.14 s in the anterior BZ and 3.63 s in the posterior BZ was found.
StdTTP of patients without cerebrovascular disease
Discussion
Perfusion measurement using DSC MR imaging contributes to evaluation of cerebrovascular occlusive disease, acute stroke, or neurodegenerative disease (2, 4, 6). Several perfusion parameters can be derived from such measurements. In addition, the mean transit time parameter TTP was postulated to be sensitive for hemodynamic impairment of cerebral perfusion in conditions of acute stroke and occlusive/stenotic cerebrovascular disease (2–4). Additionally, whereas mean transit time is computationally demanding, TTP can be derived directly from the measured curve (4).
Use of absolute TTP values obtained from a dynamic contrast/time curve with DSC MR imaging is limited because the absolute time value of TTP is influenced by many factors, such as heart ejection fraction or even the position of the venous canule. Therefore, the absolute TTP values may vary considerably inter- and intraindividually because of these peripheral effects. Introduction of an arterial input curve derived from one internal carotid artery or the proximal segment of the middle cerebral artery helps to solve this problem but is of higher technical demand on the imaging sequence and the postimaging procedures (3). From an input curve, a time offset for TTP can be defined to which all other TTP values are referenced. In this way, a major influence from peripheral effects is avoided. Drawbacks of this method are that in addition to the necessity of depicting the appropriate vessel segment by the imaging sequence, one has to be careful to take the time offset not from a vessel system mostly affected by stenosis or occlusion. The proposed stdTTP solves both problems. Because the standardization procedure searches for the point of time when the first 3% of the intracranial voxels have reached their peak enhancement in each section, the voxels corresponding to the minimally affected part of the vessel system will always supply the automatically calculated offset value for stdTTP. In patients with severest hemodynamic impairment of cerebral perfusion, with which TTP may be prolonged in a CVT, the offset calculation of stdTTP still works; this is because voxels with long TTP values cannot contribute to the offset calculation. Thus, stdTTP indicates hemodynamic stress, even in such a CVT. Furthermore, using this offset also avoids influence from peripheral effects.
Compared with other techniques, stdTTP can be considered as an intrinsic input curve derived from each imaged brain level. Performing only a simple calculation of the first enhancing voxels in each section may lead to major problems in case of artifacts (eg, liquor flow simulating signal loss in the T2*-weighted sequence). This particular situation was found in three participants in whom interference with calculation of stdTTP was sufficiently suppressed by the mask leveling procedure that was used. Therefore, the mask leveling procedure is important for using stdTTP for the hemodynamic assessment of patients. However, both procedures, mask leveling and stdTTP offset calculation, proved to be stable and reliable, which is clearly shown by the homogeneous nature of the stdTTP data from different patients. This also suggests the possibility of direct comparisons of different examinations.
Using a time resolution of 355 ms, voxels with the longest stdTTP values were considered to represent the BZs, because the contrast medium bolus being tracked by DSC MR imaging reaches voxels in CVTs earlier than in BZs. The localization of these voxels excellently matched the anatomically expected BZs in the telencephal hemispheres. Normal values for stdTTP in the anterior and posterior BZs were 3.14 and 3.63 s in participants without hemodynamic impairment in this study. Considering the different input curves, these values excellently match reports from measurements of conventional TTP using relative rescaling to other arterial segments (3).
An important impact of the time resolution of the dynamic sequence on the sensitivity of TTP to detect hemodynamic stress is known (7, 8). If the time resolution is high enough, differentiation of voxels in CVTs from those in BZs is possible with stdTTP, because stdTTP in CVTs is shorter than in BZs. This was shown in this study in that voxels in CVTs exhibited the shortest stdTTP (Fig 1), whereas voxels in the BZs expectedly showed the longest stdTTP. Because of the special offset calculation using stdTTP where the earliest enhancing voxels per section define the offset, stdTTP in CVTs without hemodynamic impairment were found to be almost equal to the offset. The relation between offset, stdTTP in CVTs, and stdTTP in BZs suggests that stdTTP is a quantitative parameter mainly reflecting the run time delay of the contrast medium bolus between CVTs and their related BZs. To take full advantage of this quality of stdTTP, which is crucial for the evaluation of hemodynamic stress, in this study, a time resolution of 355 ms was chosen. Dealing with a run time delay between the CVTs and the related BZs of approximately 3.5 s, the time resolution can be decreased to 800 ms (5). The time distance between CVTs and BZs is then depicted safely as four time steps using stdTTP. This time resolution seems to be optimal, because stdTTP examinations of the whole brain volume are possible, which are mandatory (eg, in cases of acute stroke).
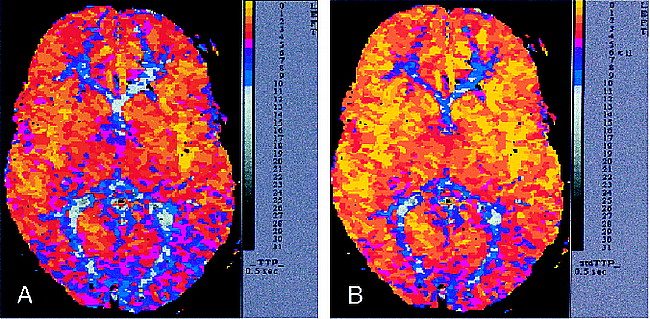
Voxels in CVTs exhibited the shortest stdTTP.
A, Conventional TTP map from an image obtained at the level of the basal ganglia by using contrast-enhanced DSC MR imaging with a T2*-weighted echo-planar fast-field-echo sequence (74/30/1; flip angle, 30°) with a 240-mm field of view, an aquisition matrix of 128 × 128 voxels, and a time resolution of 355 ms. Manual rescaling using an offset for TTP derived from the M1 segment of the left middle cerebral artery was performed to obtain an adequate perfusion map for diagnostic purposes.
B, Automatically calculated stdTTP map using the same data and scale as in panel A provides a comparable good result for TTP measurement without any loss of diagnostic information. CVTs of the anterior, middle, and posterior cerebral arteries can be differentiated as voxels with short TTP (yellow), and such voxels in BZs exhibit longer TTP (magenta and blue).
Conclusion
The stdTTP provides a quantitative measure of the hemodynamic situation of the brain with a minimum of calculatory effort and allows comparisons between different examinations when perfusion imaging is used with DSC MR imaging.
Footnotes
↵1 Address reprint requests to C. Našel, MD, Division of Neuroradiology, Department of Radiology, University of Vienna, AKH-Wien, Währingergürtel 18–20, A-1090 Vienna, Austria.
References
- Received October 12, 1999.
- Accepted after revision January 27, 2000.
- Copyright © American Society of Neuroradiology