Abstract
BACKGROUND AND PURPOSE: Perfusion MR imaging and single-photon emission CT (SPECT) are commonly used to evaluate hemodynamic status in patients with symptomatic occlusive cerebrovascular disease. These techniques rely on different underlying physiological mechanisms, and the data may not correspond. We studied the relationship between hemodynamic parameters obtained with these two methods.
METHODS: We performed perfusion MR imaging and SPECT in 10 patients with symptomatic unilateral internal carotid artery occlusion. Relative cerebral blood volume (rCBV) and uncorrected mean transit time (uMTT) were obtained with dynamic contrast-enhanced T2*-weighted MR imaging. Relative cerebral blood flow (rCBF) and vascular reserve capacity were measured with 99mTc-HMPAO SPECT; vascular reserve capacity was calculated by the difference in CBF before and after acetazolamide challenge. Ratios of these hemodynamic parameters between the affected and contralateral vascular territories were calculated and compared.
RESULTS: Normal-to-increased CBV, prolonged uMTT, decreased CBF, and normal-to-diminished vascular reserve capacity were observed in the affected vascular territories. Reduction of vascular reserve capacity corresponded well with uMTT but not with CBF and CBV. CBF, CBV, and uMTT did not correspond to one another.
CONCLUSION: uMTT is more sensitive than the other parameters in estimating vascular reserve capacity. The relationship between parameters obtained with perfusion MR imaging and SPECT should be considered in assessing the hemodynamic status of patients with symptomatic occlusive cerebrovascular disease.
Occlusion of the internal carotid artery (ICA) is a common type of chronic occlusive cerebrovascular disease that may lead to hemodynamic infarction. The hemodynamic status of the brain distal to occlusion can be assessed by various hemodynamic parameters measured with positron emission tomography (PET) (1, 2). When cerebral perfusion pressure drops, compensatory changes of the cerebrovasculature develop to maintain the normal delivery of oxygen to the ischemic brain. Increase of cerebral blood volume (CBV) and prolongation of mean transit time (MTT) occur as a result of autoregulatory vasodilatation to maintain cerebral blood flow (CBF); therefore, vascular reserve capacity (ie, residual vasodilatory capacity) diminishes along with reduction of perfusion pressure. Exhaustion of vascular reserve capacity implies hemodynamic stress at risk for stroke (3, 4).
PET cannot be applied routinely to patients, owing to its limited availability. MR imaging and single-photon emission CT (SPECT) are more accessible imaging methods than PET for evaluation of hemodynamic status of the ischemic brain in patients with chronic occlusive cerebrovascular disease. Perfusion MR imaging has been increasingly used to measure the various hemodynamic parameters in patients with carotid occlusive disease. Although this imaging method cannot easily provide absolute quantification of hemodynamic parameters, rCBV and uncorrected MTT (uMTT) can be readily calculated; an increase of CBV and a prolongation of uMTT have been described in symptomatic carotid artery occlusion (5–7). SPECT is another readily available imaging method by which to measure relative CBF (rCBF) with or without acetazolamide challenge in patients with this disease. Measurement of resting CBF alone may not provide sufficient information about hemodynamic status in chronic occlusive cerebrovascular disease and, therefore, vasoreactivity of cerebral vessels to acetazolamide has been used as an important indicator of vascular reserve capacity. Reduction of vasoreactivity to acetazolamide has been found in the affected vascular distribution (8, 9).
Perfusion MR imaging and SPECT rely on different underlying physiological mechanisms: SPECT uses freely diffusible radionuclide tracers, whereas perfusion MR imaging uses nondiffusible intravascular contrast agents. Despite this inherent difference, the hemodynamic parameters obtained from each method have been reported to correspond well to those of PET (8, 10). However, as yet, there have been no attempts to correlate the data obtained with perfusion MR imaging and SPECT. In this study, we performed both perfusion MR imaging and SPECT with acetazolamide challenge in patients with unilateral ICA occlusion. rCBV and uMTT were measured with perfusion MR imaging, and rCBF and vascular reserve capacity were calculated with SPECT. The purpose of this study was to assess the hemodynamic relationship between the parameters obtained with these two clinically available methods in the same patient group.
Methods
Patients
We prospectively examined 15 consecutive patients with symptomatic unilateral ICA occlusion during a period of 1½ years. Arterial occlusion was documented by MR angiography (n = 15) and conventional angiography (n = 6). Among these, 10 patients without large hemodynamic or territorial infarction underwent both perfusion MR imaging and SPECT. The study group included eight men and two women who ranged in age from 50 to 74 years (mean age, 60 years). Patients had experienced single or recurrent episodes of transient ischemic attack (n = 4) or reversible ischemic neurologic deficit (n = 6). 99mTc-HMPAO brain SPECT was performed within 7 days after MR imaging. Informed consent for these imaging studies was obtained from the patients or their families. All patients underwent conservative treatment during the 1-week examination period, and their symptoms were stable during this period.
Imaging Studies
MR examinations were performed with the following sequences: axial turbo spin-echo T2-weighted imaging, axial spin-echo T1-weighted imaging, MR angiography, and dynamic contrast-enhanced T2*-weighted imaging for perfusion imaging, and axial postcontrast T1-weighted imaging. The imaging parameters were 3500/90 (TR/TE) for T2-weighted sequences and 550/14 for pre- and postcontrast T1-weighted sequences. Section thickness was 5 to 6 mm and the matrix was 200 × 256. MR angiography was performed around the circle of Willis in the axial plane, and at the level of the cervical carotid artery in the coronal plane, with a standard 3D time-of-flight sequence (43/8, 20° flip angle, 64-mm slab thickness, and 256 × 512 matrix).
Dynamic contrast-enhanced T2*-weighted imaging was performed with a conventional gradient-echo sequence (40/26, 10° flip angle, 64 × 128 matrix, 5- to 6-mm slice thickness, and 3.8-second acquisition time). Between 17 and 20 single-section dynamic images were obtained at the level of the upper basal ganglia. After acquisition of three images, gadodiamide was administered manually via the forearm vein as a bolus within 5 seconds, followed by a flush of 30 mL saline. The total imaging time was approximately 60 seconds after initiation of the bolus injection.
SPECT studies for rCBF and vascular reserve capacity were performed by using a triple-head gamma camera system with fanbeam collimator (Multi-SPECT3, Siemens, Chicago, IL) within 7 days after the MR examinations. Patients received 20 mCi (740 MBq) of 99mTc-HMPAO and, 5 minutes later, the first scan was started (acquisition time, 20 minutes) for CBF imaging (ie, resting CBF) (Fig 1). At 8 minutes after the start of the first scan, 1 g of acetazolamide was administered. At the end of the first scan, 30 mCi (1110 MBq) of 99mTc-HMPAO was injected again, and the second scan (acquisition time, 20 minutes) was started 5 minutes later for postacetazolamide CBF imaging. Images were reconstructed in a 128 × 128 matrix (9.4 × 9.7-mm in-plane resolution) with a 5-mm slice thickness in transverse, sagittal, and coronal planes using Butterworth filtered backprojection.
Scanning protocol for SPECT. At 5 minutes after injection of the first dose of 99mTc-HMPAO, the first scan (acquisition time, 20 minutes) starts for resting CBF imaging. At 8 minutes after the start of the first scan (ie, 12 minutes before the second HMPAO injection), 1 g of acetazolamide is administered. At the end of the first scan, the second dose of 99mTc-HMPAO is injected, and the second scan for postacetazolamide CBF imaging starts 5 minutes later
Data Analysis
All dynamic MR images were transferred via Ethernet to a personal computer after image acquisition and were evaluated with the use of in-house software. Only rCBV and uMTT maps were created, because deconvolution of arterial input function was not available for this study. In accordance with the indicator dilution theory, the nonlinear regression method was used to fit a gamma-variate function to the contrast material concentration versus time curve on a pixel-by-pixel basis, under the assumption of an exponential relationship between relative signal reduction and contrast material concentration (11). Then, CBV was calculated by numerical integration of the area under the concentration-time curve (ie, CBV = ∫ C(t)dt), and uMTT was calculated as the ratio of the first moment of the concentration-time curve and CBV (ie, uMTT = ∫ tC(t)dt/∫ C(t)dt) (12). On the CBV and uMTT maps, an irregular but mirror-shaped region-of-interest (ROI) was placed in the whole middle cerebral artery (MCA) territory of the affected hemisphere and in the corresponding contralateral region. Lesion-to-contralateral CBV and uMTT ratios (ie, CBV or uMTT in the affected MCA territory divided by that of the corresponding contralateral region) were then calculated.
To analyze the SPECT scans, we selected three consecutive transverse images that included the plane of the perfusion MR imaging. Symmetrical mirror ROIs were placed on each of the selected images, as in measurements of CBV and uMTT ratios. Lesion-to-contralateral CBF ratios (ie, radioisotope activity in the affected MCA territory divided by that of the corresponding contralateral region) were then calculated on the three sections of both CBF and postacetazolamide CBF images, and those ratios were averaged. Vascular reserve capacity was expressed as δCBF ratio (CBF ratio minus postacetazolamide CBF ratio) in a similar manner to that reported previously (8). Therefore, the larger value of δCBF ratio indicates the more diminished vascular reserve capacity.
To assess the relationship between these hemodynamic parameters obtained with perfusion MR imaging and SPECT, we compared CBV ratios, uMTT ratios, CBF ratios, and δCBF ratios by using the Spearman correlation coefficient. Statistical computations were performed with the SPSS statistical software package (SPSS, Chicago, IL), and the level of significance was defined as P < .05.
Results
Patient and hemodynamic data are summarized in the Table 1. ICA occlusion was found at the origin of the ICA in nine patients and at the distal supraclinoid ICA in one patient. The MCA supply in the affected hemisphere was via the anterior and/or posterior communicating artery in nine patients and via the ipsilateral ophthalmic artery in one patient. T2-weighted images showed multiple small areas of high signal intensity predominantly in the affected centrum semiovale in seven patients and symmetrically distributed in both centra semiovale in two patients; one patient had no abnormal signal.
Summary of findings
From perfusion MR imaging data, CBV in the affected MCA territory was normal to increased in all 10 patients as compared with that of the corresponding contralateral region (Fig 2A–C), and CBV ratios were 1.00 to 1.39 (median, 1.03) (Fig 2D). The uMTT in the affected vascular territory was prolonged in all 10 patients (Fig 2E), and those ratios were 1.01 to 1.13 (median, 1.07). From SPECT data, CBF in the affected vascular territory decreased in all 10 patients (Fig 2F), and those ratios were 0.81 to 0.98 (median, 0.94). After acetazolamide administration, the CBF difference between the affected vascular territory and the corresponding contralateral region was more pronounced in nine patients (Fig 2G) but not in one patient. Postacetazolamide CBF ratios were 0.69 to 0.97 (median, 0.89), and therefore δCBF ratios were 0 to 0.14 (median, 0.05).
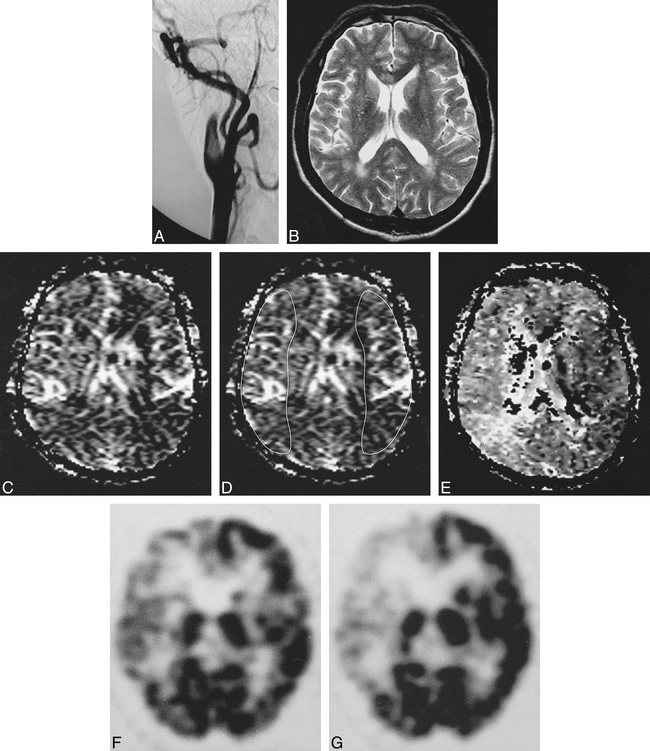
Case 1: 59-year-old man with reversible ischemic neurologic deficit.
A, Anteroposterior view of right common carotid angiogram shows complete occlusion of the right proximal ICA.
B, T2-weighted image (3500/90/1) obtained at the level of perfusion MR imaging is normal except for subtle ischemic lesions in both occipital periventricular white matters.
C, CBV map shows slightly increased CBV in the right whole MCA distribution compared with contralateral corresponding region.
D, CBV map shows irregular ROIs placed for measurement of CBV ratio between the affected MCA territory and the contralateral region. Calculated CBV ratio was 1.04.
E, Corresponding uMTT map shows increased uMTT in the right whole MCA distribution compared with contralateral region.
F, 99mTc-HMPAO brain SPECT scan obtained at approximately the same level as C and E reveals mild hypoperfusion throughout the right whole anterior and MCA territories.
G, 99mTc-HMPAO brain SPECT scan with acetazolamide challenge reveals remarkable hypoperfusion in the right side as compared with F, suggesting reduced vascular reserve capacity in the right side.
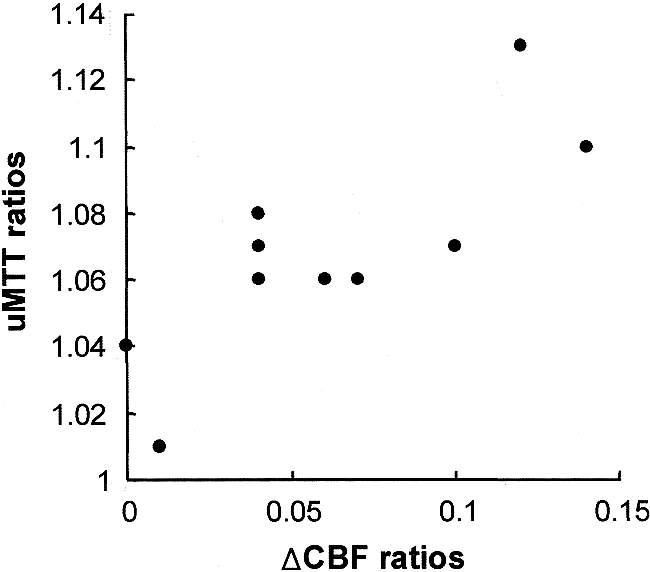
The δCBF ratios correlated well with the MTT ratios (r = .69, P < .05) (Fig 3) but not with the CBF ratios (r = −.28, P > .05) or CBV ratios (r = −.35, P > .05). The CBF, CBV, and MTT ratios did not correlate with one another (r = .52 and P > .05 between CBF and CBV ratios; r = −.36 and P > .05 between CBF and MTT ratios; r = −.14, P > .05 between CBV and MTT ratios).
Scatterplot shows the relationship between uMTT ratios and δCBF ratios. There is a significant correlation between uMTT ratios and δCBF ratios (r = 0.69, P < .05)
Discussion
Various imaging methods have been used to obtain hemodynamic information about chronic occlusive cerebrovascular disease, and therefore, to identify patients under hemodynamic stress (ie, at risk for stroke). Although PET is the most reliable method for providing hemodynamic (ie, CBF, CBV, and MTT) as well as metabolic (ie, oxygen extraction fraction and oxygen utilization) data (3, 13), perfusion MR imaging and SPECT are more accessible than PET in the clinical arena. rCBV and uMTT values can be readily calculated with perfusion MR imaging (5), and rCBF and vascular reserve capacity are easily obtainable with SPECT (9, 14). Despite the increasing application of perfusion MR imaging and SPECT to chronic occlusive cerebrovascular disease, as yet there has been no correlative study to clarify the relationship between the hemodynamic parameters obtained with the two methods.
Cerebral perfusion pressure in a given arterial territory is the pressure difference between mean arterial pressure and venous pressure. When the perfusion pressure increases or decreases, compensatory vasoconstriction or vasodilatation occurs as a result of autoregulation to maintain normal blood flow (15). As a result of autoregulatory vasodilatation in chronic occlusive cerebrovascular disease, an increase of CBV and a prolongation of MTT occur in the brain tissue distal to the occlusion to maintain CBF, as in this study. With further reduction of perfusion pressure, vasodilatation becomes maximal and then CBF begins to decrease. Therefore, a severe reduction of perfusion pressure means exhaustion of vascular reserve capacity (ie, residual vasodilatory capacity), which may be the most important criterion for selecting candidates for extracranial-intracranial bypass surgery (3). According to previous PET studies, however, measurements of either CBF or CBV alone cannot estimate vascular reserve capacity precisely because they do not change linearly to reduced perfusion pressure (3, 13). In contrast, the CBV/CBF ratio (ie, MTT) has been reported to correlate with reduced vascular reserve capacity better than CBV or CBF alone (3, 8, 13).
Among the patients in this study, normal-to-increased CBV, prolonged uMTT, decreased CBF, and normal-to-diminished vascular reserve capacity were observed in the affected vascular territories. Although CBF, CBV, uMTT, and vascular reserve capacity in this study were not obtained from a single imaging method, we found a close correlation between uMTT prolongation and reduced vascular reserve capacity, which is consistent with previous results, as mentioned above. Our finding that neither CBF nor CBV alone correlates with uMTT or vascular reserve capacity supports the theory that measurement of only CBF or CBV is not sufficient to estimate vascular reserve capacity. Moreover, the lack of correlation between CBF and CBV in this study also suggests that neither parameter changes linearly in response to reduced perfusion pressure. These results support the idea that uMTT is the parameter that responds the most linearly to reduced perfusion pressure, as described in previous PET studies (3, 13).
Identification of patients at risk for hemodynamic infarction (ie, at the stage of exhausted vascular reserve capacity) is important in chronic occlusive cerebrovascular disease, because extracranial-intracranial bypass surgery may be beneficial for these patients. Assessment of vascular reserve capacity has been conducted by measuring the oxygen extraction fraction and the CBV/CBF ratio (ie, MTT) using PET (3, 13) and by determining vasoreactivity to acetazolamide using SPECT or xenon CT (7, 8). Because of limited availability of PET, vasoreactivity to acetazolamide on SPECT or xenon CT has been more widely used than PET (9, 14, 16). The mechanism by which acetazolamide dilates cerebral vessels is unknown, but direct vasodilatory action or secondary metabolic change due to carbonic anhydrase inhibition have been described (17, 18). Although vasoreactivity to acetazolamide was reported to be variable among patients (19, 20), it is known to correlate well with oxygen extraction fraction and MTT measured with PET (8). Recently, perfusion MR imaging studies with acetazolamide have been performed in healthy volunteers and in patients with occlusive cerebrovascular disease, and have provided promising results (21–23). However, the use of acetazolamide with perfusion MR imaging should be validated in studies with a greater number of patients and the results carefully compared with the data obtained from other established imaging techniques, especially PET. In this study, we assessed vascular reserve capacity using a method similar to that reported by Hirano et al (8), calculating the difference in CBF before and after the use of acetazolamide with SPECT. All symptomatic patients except one in this study had reduced vascular reserve capacity on SPECT, and all had prolonged uMTT on perfusion MR imaging. The two parameters correlated well. However, there is no consensus about the degree of reduction of vascular reserve capacity and of prolongation of MTT in selecting candidates for bypass surgery. Further studies are needed for comprehensive analysis of hemodynamic parameters, clinical signs and symptoms, and patient outcomes after treatment to determine the selection criteria (24).
This study has several limitations. First, because there were no normative data from healthy volunteers for perfusion MR imaging and SPECT, we could not provide the reference values to determine how much asymmetry in the parameter ratios in the patients should be interpreted as abnormal. Although minimally decreased CBF and minimally increased CBV might be within the normal range, we think that the correlation patterns found between the various hemodynamic parameters in this study were of clinical significance to the interpretation of the perfusion MR imaging and SPECT data. Second, there are different underlying physiological mechanisms between perfusion MR imaging and SPECT. SPECT uses freely diffusible tracers so that the entire tissue volume is measured, whereas perfusion MR imaging uses contrast agents that stay within the intravascular space. Furthermore, perfusion MR imaging depends on several factors, such as contrast injection rate, patients' cardiac output, and different rates of contrast passage between the various locations of the brain, all of which diminish the accuracy of the data (25). Thus, the data obtained with the two methods may not be exactly interchangeable. Nevertheless, the hemodynamic parameters obtained from each method correlated well with those of PET (8, 10), and therefore our attempt to correlate the data between perfusion MR imaging and SPECT can be considered an expansion of clinical applicability. Third, only single sections can be evaluated with the conventional dynamic contrast-enhanced T2*-weighted imaging technique. Thus, concerns may be raised about the reliability of data obtained from a single section without covering the whole area of ischemia. This limitation could be mitigated by using an echo-planar imaging technique with the capability of superior temporal resolution and therefore multislice imaging. Fourth, the uMTT measured with perfusion MR imaging in this study is not equal to true MTT because it was calculated from the first moment of the concentration-time curve. However, ratios of the first moments has been used to provide a relative measure of MTT (5, 7). More accurate assessment of MTT requires multislice imaging with measurement of arterial input function, faster bolus injection of contrast material, and specialized image processing software for deconvolution of arterial input function (26, 27), although arterial input data obtained noninvasively with current MR imaging techniques are less accurate than those with radionuclide studies in which invasive arterial sampling and kinetic analysis are possible (28).
Conclusion
In this study, we measured rCBV and uMTT with perfusion MR imaging and rCBF and vascular reserve capacity with SPECT in a small group of patients with unilateral ICA occlusion. We found that vascular reserve capacity correlated closely with uMTT but not with CBF or CBV. CBF, CBV, and uMTT did not correlate with one another. Although these hemodynamic parameters were not obtained from a single imaging method, our results support previous findings that CBF and CBV do not change linearly in response to reduced perfusion pressure, and that uMTT is more sensitive than the other parameters in estimating vascular reserve capacity. This hemodynamic relationship should be considered in assessing the hemodynamic status of chronic occlusive cerebrovascular disease with perfusion MR imaging and/or SPECT.
Footnotes
1 Supported by grant HMP-97-NM-2-0038 from the Good Health R & D Project, Ministry of Health and Welfare, South Korea.
↵2 Address reprint requests to Jae Hyoung Kim, MD, Department of Radiology, Gyeongsang National University Hospital, 90 Chilam-dong, Chinju 660–702, South Korea.
References
- Received November 15, 1999.
- Accepted after revision February 17, 2000.
- Copyright © American Society of Neuroradiology