Abstract
Summary: We report a case of a 19-year-old woman who underwent radiosurgical treatment of a residual arteriovenous malformation. Nine months after treatment, repeat angiography revealed a de novo paranidal aneurysm that was treated endovascularly. We postulate that changes in flow dynamics or vessel integrity after radiosurgery contributed to the formation of her de novo aneurysm.
Stereotactic radiosurgery is a well-established treatment option for arteriovenous malformations (AVMs). The potential complications related to radiosurgery are well documented and are predominately related to radiation effects to the surrounding brain parenchyma (1, 2). Following treatment, complete obliteration of a vascular lesion may not occur for several years. During this latency period, the patient remains at risk for hemorrhage. Despite concerns for increased risk of hemorrhage during the early experience with radiosurgery, subsequent studies have shown that the hemorrhage rate is comparable to the natural history of these lesions (3, 4).
This report describes a patient who underwent a subtotal resection of a large left hemispheric AVM followed by gamma knife treatment of her residual nidus. Nine months after treatment, she developed increased seizures associated with an episodic postictal hemiparesis. Repeat angiography showed a de novo paranidal aneurysm arising from a distal anterior cerebral artery branch supplying her residual nidus. This case suggests that, in certain circumstances, changes following radiosurgery may predispose to aneurysm formation in feeding vessels.
Case Report
This 19-year-old right-handed woman presented with a left frontal intraparenchymal hemorrhage and a right hemiplegia 2 years before the writing of this report. Her workup revealed a large left frontoparietal AVM. The patient subsequently underwent preoperative embolization followed by a microsurgical resection of her lesion at an outside institution. Her family declined immediate postoperative angiography.
The patient recovered well and was able to ambulate independently with a mildly spastic gait 1 year after her procedure. A follow-up angiogram was eventually obtained that revealed a small residual nidus (Fig 1), and the patient underwent gamma knife treatment of the nidus. The total volume of the lesion was 0.25 cm3. The margin of the nidus was covered by the 50% isodose line, and 25 Gy were delivered to the margin.
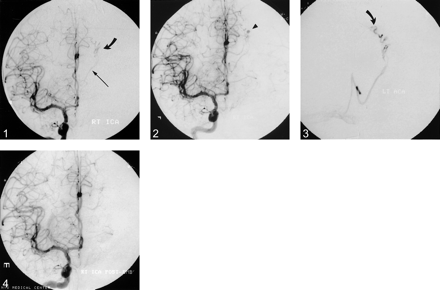
Arterial phase image (frontal projection) from a digital subtraction angiogram of the right internal carotid artery acquired at the time of radiosurgical planning. Note the small residual nidus (curved arrow) of a left posterior frontal AVM. The nidus is supplied by a posterior internal frontal branch of the left anterior cerebral artery and drains into the left thalamostriate vein (thin arrow). Owing to a hypoplastic left A1 segment, the AVM was not well visualized by left internal carotid artery angiography.
fig 2. Right internal carotid artery angiogram (frontal projection) 9 months later disclosing the interval development of a small paranidal aneurysm (arrowhead)
fig 3. Superselective digital subtraction angiogram (frontal projection) obtained prior to embolization of the aneurysm and nidus with n-butyl-cyanoacrylate. The microcatheter tip is indicated by the curved arrow.
fig 4. Postembolization right internal carotid artery angiogram (frontal projection) confirming occlusion of the aneurysm
Nine months after her treatment, the patient developed intermittant sensory seizures. MR imaging suggested the presence of a new aneurysm. Repeat angiography revealed a reduction in flow to the nidus and confirmed the presence of a de novo paranidal aneurysm arising from a distal pericallosal branch vessel supplying the residual nidus (Figs 2 and 3). The feeding vessel was subsequently embolized and the nidus was obliterated (Fig 4).
Discussion
Microsurgical resection remains the most effective treatment for AVMs of the brain. However, depending on the size and location of the lesion, surgery may carry an unacceptable risk of serious neurologic sequela. Over the past several years, the development of endovascular and radiosurgical techniques have improved our ability to treat lesions that were previously considered untreatable.
The advantages and disadvantages of stereotactic radiosurgery for brain AVMs have been discussed extensively in the literature (4–6). Complete obliteration of an AVM after radiosurgery is not immediate. During this latency period, the patient remains at risk for hemorrhage. The hemorrhage rate during this latency is similar to the rate of hemorrhage in untreated AVMs (3, 4). However, the presence of intranidal aneurysms or venous outflow stenosis may increase the near-term risk of hemorrhage. Radiosurgical treatment of lesions with these angioarchitectural features may carry a higher risk of hemorrhage before complete obliteration is achieved.
Three types of aneurysms are associated with cerebral AVMs: flow-related aneurysms, intranidal aneurysms, and congenital aneurysms. Flow-related aneurysms are probably acquired lesions and typically involve a vascular root supplying the AVM. They presumably result from increased shear stress along the vessel wall due to the hyperdynamic flow through the arteriovenous shunt. They occur in multiple locations in over half of patients affected.
Intranidal aneurysms occur in 5.5% to 58% of AVMs (7, 8). They are frequently multiple and usually less than 4 to 5 mm in size. These aneurysms typically arise from a feeding artery as it merges into the nidus. These aneurysms exhibit a thin wall of 3 to 4 cell layers, scant smooth muscle, and no elastic fibers. They are associated with an increased incidence of hemorrhage (8) and may occur in approximately 40% of patients with intracranial hemorrhage related to an AVM.
There are several possible explanations for the de novo formation of a paranidal aneurysm in our patient. Potentially, a preferential radiotherapeutic effect on venular vascular structures within the nidus could have increased venous outflow impedence, thereby increasing intraluminal pressure within the supplying arteries proximal to the AVM nidus. Increased cerebral vessel diameter within targeted radiation fields has been demonstrated in experimental settings (9). The pathogenesis of this phenomenon has been ascribed to downstream microvascular occlusion or impaired venous drainage acting to increase intraluminal pressures within the upstream microvasculature. It seems reasonable that a reduction in flow through the nidus could result in increased intramural pressure in the feeding artery and subsequent aneurysm formation.
Alternatively, a focal vasculopathic effect of the radiation on the vessel wall could have led to aneurysm formation (10). The location of the aneurysm was in close proximity to the treatment margins. Retrospective review of the treatment plan showed that the feeding vessel received 20 Gy of radiation at the site of aneurysm formation. Doses as low as 5–9 Gy may be sufficient to cause changes in normal vessels (11). Radiation effects to blood vessels result in intimal hyperplasia and stenosis (9), although these specific structural changes are not known to increase the risk of aneurysm formation. Finally, formation of the aneurysm in our patient may have been part of the natural history of her lesion, independent of her treatment.
It is difficult to know, from a single case report, what set of factors or circumstances led to aneurysm formation in our patient. We were fortunate in documenting the progression of these events before a new rupture. It seems reasonable that changes in flow dynamics and/or vasculopathic effects after radiation treatment may, in certain circumstances, predispose these patients to an increased risk of rupture. However, it is doubtful that this is a common occurrence since the overall hemorrhage rate after treatment is comparable to the hemorrhage rate of untreated lesions.
Footnotes
1 Address reprint requests to Paul P. Huang, MD, Department of Neurosurgery, NYU Medical Center, 530 First Ave., Suite 8R, New York, NY 10016.
References
- Received December 15, 2000.
- Copyright © American Society of Neuroradiology