Abstract
BACKGROUND AND PURPOSE: Alterations in intra-aneurysmal pressure and flow have been observed after treatment with Guglielmi detachable coils (GDCs). We wished to determine whether these changes could be assigned to a hydrodynamic effect of the coils themselves or a compound effect of coils plus thrombus formation.
METHODS: Intra-aneurysmal pressure and flow were measured with a 0.014-inch guidewire- mounted transducer in a canine aneurysm in vivo and in vitro before and after treatment with GDCs. Flow was evaluated by using the thermodilution technique. Pressure and flow were also recorded in a bifurcational silicone aneurysm mounted onto a flow phantom during variations in systemic pressure and pulse rate before and following the insertion of GDCs.
RESULTS: The insertion of GDCs induced a reduction in flow that was qualitatively similar when the aneurysm was perfused either by blood (in vivo) or with normal saline (in vitro). Quantitatively, however, flow was reduced less distinctly during perfusion with saline. In the silicone aneurysm, pressure was inversely related to pulse rate and increased with augmenting systemic pressure, whereas flow remained constant regardless of variations in pressure and pulse rate. After GDC placement, reduced flow was dependent on pulse rate but independent of systemic pressure.
CONCLUSION: GDCs significantly reduced flow even in the absence of thrombus, indicating that they have a purely hydrodynamic effect. In the silicone model, the decrease in intra-aneurysmal flow after coiling relied upon the pulse rate in a manner suggesting the presence of resonance phenomena.
Following successful coiling of ruptured aneurysms with the Guglielmi detachable coil (GDC), there is an immediate and durable reduction in the incidence of aneurysm rebleeding at least similar in magnitude to that seen following aneurysm clipping (1). Although it has been demonstrated, both in vivo and in vitro, that coiling induces immediate alterations in intra-aneurysmal pressure and flow that serve to reduce hemodynamic stresses (2–5), it remains unclear whether these changes are caused by the mere presence of the coils themselves or are the result of the coils plus thrombus that has formed during and immediately after treatment.
The aim of our study was to determine whether or not changes in pressure and flow observed after coiling were the result of the presence of coils plus thrombus (what might be called a hemodynamic effect) or simply the result of the presence of the coils themselves (what might be called a hydrodynamic effect). It was our hypothesis that, immediately after the insertion of coils, a hydrodynamic effect reduces stresses on the aneurysm wall. The immediate protective effects derived from coils is likely due to their distribution in an aneurysm as much as the final density of coil packing that is achieved.
The genesis, growth, and eventual rupture of saccular intracranial aneurysms seem to be closely associated with hemodynamic forces (6). The hemodynamic behavior within aneurysms has been previously described (2–5, 7). The effect of changes in systemic pressure and heart frequency on intra-aneurysmal flow, however, is not well documented. Changes in systemic pressure and heart rate might have impact on the coiled aneurysm, potentially affecting intra-aneurysmal pressure and residual flow. Such changes could represent promoters for coil compaction, further growth of the aneurysm after coiling, and, at worst, rerupture of the coiled aneurysm. To this end, we also wished to delineate the effect of changes in systemic pressure and pulse rate on intra-aneurysmal pressure and flow before and after treatment with GDCs by means of a perfusion model.
Methods
As we previously observed, the insertion of a GDC into an aneurysm causes alterations of the intra-aneurysmal pressure and flow (2, 3). In particular, there occurs a marked decrease in flow within the aneurysm after insertion of merely one single coil (3). Whether this stagnation of flow can be assigned to a hydrodynamic effect of the coils themselves (similar to a breakwater) or if it is simply the result of thrombus formation remains unclear. Therefore, we wished to observe which alterations in pressure and flow occur after coiling of the very same aneurysm when it is perfused with blood (in vivo) versus when the same aneurysm is perfused by saline (in vitro). With the following study design we can observe pressure and flow in the dome of the aneurysm after coiling in an identical setting apart from excluding the possibility of thrombus formation in the in vitro situation. In the latter, the pure hydrodynamic effect of coils would emerge.
In Vivo Experiment
Under an institutional animal use protocol, we created a side-wall aneurysm in a mongrel dog by using a technique originally described by German and Black (8) and later modified in our laboratory (9). This aneurysm was studied for 6 months after it was created. Endotracheal anesthesia was achieved with halothane, and the dog was given 1000 IU of heparin. We placed a 6F vascular sheath into both common femoral arteries by means of a cut-down. Through one of the vascular sheaths, a 6F guiding catheter was positioned proximal to the aneurysm in the common carotid artery. Fluoroscopy and digital subtraction angiography were performed by using a portable C-arm. Thereafter, a 0.014-inch guidewire-mounted pressure-temperature sensor (PressureWire Sensor; RADI Medical Systems, Uppsala, Sweden) was calibrated to zero at room temperature and at the barometric room pressure. The sensor was positioned at the dome of the aneurysm and kept stable in this position by the 3-cm-long distal flexible tip of the pressure-temperature guidewire. Using a commercially available angiographic injector (Angiomat 3000; Liebel-Flarsheim Company, Cincinnati, OH), we injected normal saline at room temperature through the guiding catheter 6–8 cm proximal to the aneurysm at rates of 5 mL/s over 4 seconds and 15 mL/s over 2 seconds. Injections of this type induce abrupt and reproducible increases in intravascular pressure (2). This technique also allows, with the principle of thermodilution, assessment of flow within the aneurysm; the observed decrease in temperature is proportional to the volume flow rate at the site of measurement (3). The measured intravascular pressures and thermodilution curves were recorded by using Run-Time LabVIEW 5.1 software (National Instruments, Austin, TX).
The heart rate in the dog varied between 100 and 120 beats per minute, while the arterial mean blood pressure varied between 80 and 120 mm Hg. We performed five consecutive injections with room-temperature normal saline at each rate of either 5 mL/s over 4 seconds or 15 mL/s over 2 seconds. With the pressure-temperature wire in place, a Tracker Catheter (Target Therapeutics/Boston Scientific, Natick, MA) was introduced into the aneurysm, and GDCs were filled into the aneurysm and detached by using standard clinical techniques. Coiling was considered complete when no more coils could be introduced and the parent artery remained uncompromised. After completed coiling, intra-aneurysmal pressure and flow once more were evaluated by administering five consecutive injections of normal saline at each of the rates mentioned earlier. After all measurements were completed, the dog was euthanized by using Beuthanasia D, 1 mL/5 lbs intravenously. Thereafter, the carotid artery with the aneurysm was resected, and the mass of GDCs with the pressure-temperature wire carefully removed. The specimen was then immediately irrigated with a physiologic solution to remove any blood or thrombus.
In Vitro Experiment
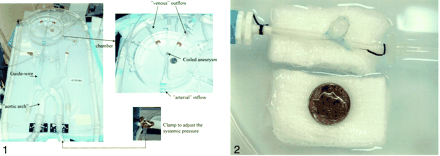
To study the explanted aneurysm, we used a custom-made system comprising a molded silicone replica of the aortic arch and brachycephalic vessels. Fitted to segments representing the internal carotid arteries and vertebral arteries were connections leading to a chamber into which the explanted aneurysm could be placed and connected to the systemic arterial and venous circulation (MicroVention, Inc, Aliso Viejo, CA) (Fig 1). The flow phantom was connected to an electromagnetic flow pump (LMI Milton Roy, Acton, MA) that allowed pulsatile perfusion with pulse rates between 40 and 100 beats per minute. The systemic pressure could be varied by adjusting a clamp mounted on the main inflow vessel (Fig 1). The model was perfused with a normal saline and glycerol solution that maintained a constant temperature of 37°C controlled by a thermostat-heater control unit.
Vascular silicone phantom with a replica of the aortic arch, brachycephalic vessels, and a cerebral basin (upper insert). The systemic pressure could be adjusted by using a clamp on the main inflow vessel (lower insert).
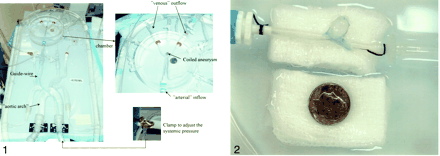
The explanted specimen was mounted onto the inflow and outflow connections in the basin by using a watertight technique (Fig 2). A 6F guiding catheter was positioned close to the entrance to the cerebral basin. The 0.014-inch guidewire-mounted pressure-temperature sensor was recalibrated as described before and inserted into the canine aneurysm at the same site as it was when used in vivo. The pulse rate of theelectromagnetic pump was set to 100 beats per minute, and the mean systemic pressure was set in the same range as in the canine blood pressure. As in the in vivo experiment, five injections with normal saline at room temperature at a rate of 5 mL/s over 4 seconds as well as 15 mL/s over 2 seconds were performed. A Tracker Catheter (Target Therapeutics/Boston Scientific) was advanced into the aneurysm under fluoroscopic guidance. Coiling was then carried out by using techniques identical to those of the in vivo study (size, number, and sequence of coils). Following complete coiling, measurements of intra-aneurysmal pressure and flow were again made by using five consecutive injections with normal saline at room temperature at rates identical to those already described.
Surgically created canine aneurysm after explantation mounted onto the inflow and outflow connections in the basin of the vascular model.
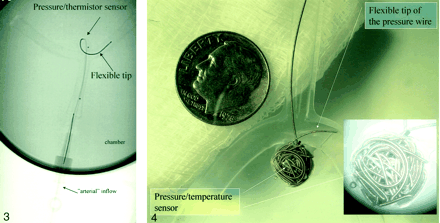
When all measurements of the canine aneurysm were completed, it was removed, and a silicone bifurcational aneurysm replica was mounted into the basin. The 6F guiding catheter remained in place proximal to the parent artery. Through this guiding catheter, another 0.014-inch pressure-temperature sensor was positioned in the dome of the bifurcation aneurysm (Fig 3). Five consecutive saline injections at a rate of 5 mL/s over 4 seconds were performed at various combinations of pulse rate (40, 60, 80, and 100 beats per minute) and systemic pressure (60, 95, 120, and 150 mm Hg), resulting in a total of 80 injections (5 × 4 pulse rates × four pressures). After each injection with normal saline at room temperature, the system temperature was allowed to return to 37°C. Thereafter, with the pressure-temperature wire in place, a Tracker Catheter (Target Therapeutics/Boston Scientific) was introduced into the aneurysm and GDCs were positioned and detached as described before. The aneurysm was maximally packed with GDCs following clinical and angiographic criteria (Fig 4). When coiling was completed, the 80 injections of room temperature saline at various pulse rates, and pressures were repeated in a manner identical to that done before coiling.
The bifurcational silicone aneurysm with the 0.014-inch pressure-temperature sensor guidewire in place at the dome. The aneurysm is mounted in the basin of the vascular model. Note the flexible tip distal to the sensor that helps to maintain a stable placement of the sensor.
Bifurcational silicone aneurysm after the insertion of GDCs. The 0.014-inch pressure-temperature sensor guidewire is still in place (arrow). The insert shows the coiled aneurysm through the microscope.
Data Analysis
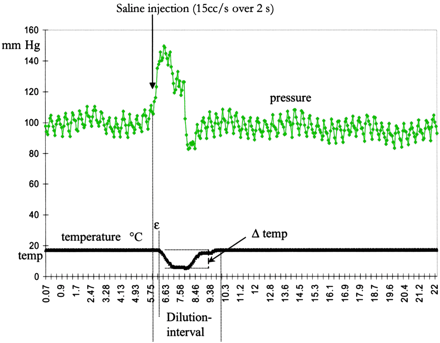
The data were analyzed with respect to the increase in pressure induced by the injections with normal saline as well as the following thermodilution parameters: maximum decrease in temperature caused by the saline injections (ΔT) and the dissociation of pressure and flow (which exists in all pulsatile systems) denoted as shift (ε) that is, the time gap between the observed increase in pressure and the start of decrease in temperature. The observed total length of temperature change induced by the saline injection was denoted as the dilution interval (Fig 5).
Example of the increase in pressure in millimeters of mercury (top) and thermodilution curve in degrees Celsius (bottom) obtained from an injection of normal saline 15 mL/s over 2 seconds at room temperature. Dilution interval denotes the entire period of temperature change, ΔT represents the largest drop in temperature achieved by injection of the saline, and ε is the gap between the start of pressure increase and the start of fall in temperature (dissociation of pressure and flow)
The five repetitive injections at each combination of pulse rate and pressure were averaged and plotted against the specific frequency. SPSS, version 10.0 (SPSS Inc, Chicago, IL) was used for statistical analysis. For statistical analysis of intra-aneurysmal pressure and flow at varying pulse rates and systemic pressures, we used the linear-term contrast one-way analysis of variance, with the exception of the comparison of the variables before GDC treatment versus after GDC treatment for which we used t statistics (two-tailed paired-samples test).
Results
Effect of GDCs in Vivo versus in Vitro
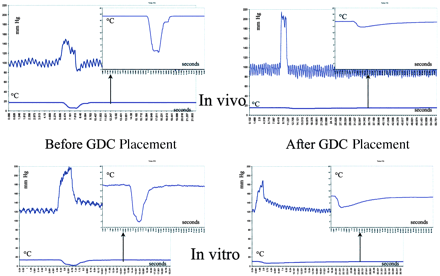
The results of the injections of normal saline at room temperature at a rate of 15 mL/s over 2 seconds are summarized in Table 1, whereas the corresponding results for the injection rate of 5 mL/s over 4 seconds are shown in Table 2. We observed characteristic increases in pressure and the typical U-shaped thermodilution curve both in vivo and in vitro (Fig 6). No significant differences in the characteristics of the thermodilution curves were observed before treatment with GDCs (Tables 1 and 2). Before the insertion of GDCs, the increases in pressure obtained by the injections with normal saline were higher in vitro than in vivo.
Pressure and thermodilution curves in the canine aneurysm in vivo (top) and in vitro (bottom) before (left) and following (right) placement of GDCs. The inserts represent magnifications of the respective thermodilution curves. Before coiling, there are similar U-shaped thermodilution curves both in vivo and in vitro. After coiling, the thermodilution curves are flattened considerably both in vivo and in vitro, but more markedly in vivo.
Pressure and flow in the dome of a surgically created canine aneurysm, injection at 15 mL/s over 2 seconds
Pressure and flow in the dome of a surgically created canine aneurysm, injection at 5 mL/s over 4 seconds
After placement of the GDCs, the increases in pressure due to the saline injections were not attenuated when the aneurysm was studied in vivo (Tables 1 and 2). In vitro, the corresponding increases in pressure were at both injection rates significantly diminished after coiling.
The placement of GDCs altered the thermodilution curves in a similar manner both in vivo and in vitro (Fig 6). At an injection rate of 15 mL/s over 2 seconds there were no significant differences in hemodynamics versus hydrodynamics apart from a smaller dissociation of pressure and flow (ε) in vitro (Table 1). The signs of flow stagnation were generally more accentuated when the aneurysm was perfused with blood than with saline. At the injection rate of 5 mL/s over 4 seconds, both the pressure-flow shift ε, the length of the dilution interval, and the maximum change in temperature were less dramatically changed by the placement of GDCs in vitro; that is, the signs of flow stagnation were quantitatively less distinct (Table 2). However, qualitatively, the flow changes observed after the insertion of GDCs were similar at perfusion with blood as compared with perfusion with saline; that is, the maximum change in temperature is significantly reduced (ie, reduced inflow into the aneurysm), the pressure-flow shift is increased (ie, increasing dissociation of pressure to flow), and the dilution interval is prolonged (ie, decreased turnover of the fluid).
Intra-Aneurysmal Pressure Increase
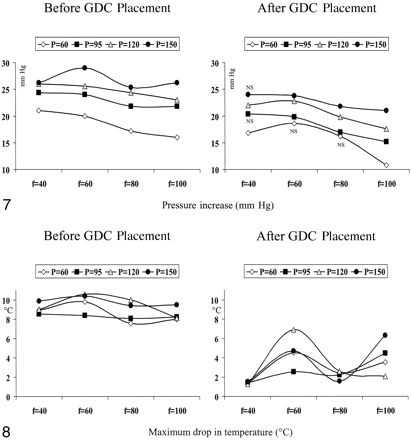
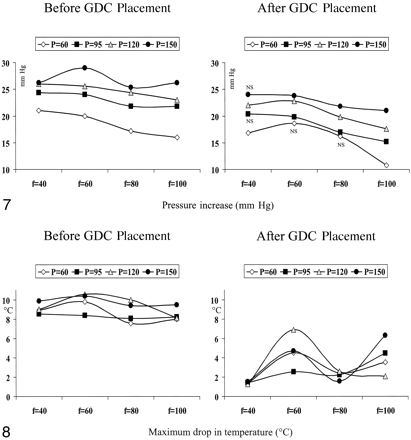
The intra-aneurysmal increase in pressure to the injections of saline was augmented with increasing systemic pressure at all pulse rates (P < .01 for all pulse rates, Fig 7). On the contrary, the intra-aneurysmal pressure increase diminished with increasing pulse rate at all pressure levels (P < .01 at 60 mm Hg, P < .05 at all other pressures). Treatment with GDCs attenuated the increase in intra-aneurysmal pressure obtained by the injections with saline (P < .03). However, this attenuation was statistically significant in only half of the conditions when either the pulse rate was low or the systemic pressure was low (Fig 7).
Increase in intra-aneurysmal pressure caused by the injection of normal saline at a rate of 5 mL/s over 4 seconds at various systemic pressures (P) and pulse rates (f), both before (left) and after (right) the insertion of GDCs. The pressure increases augmented with increasing systemic pressure and decreasing pulse rate.
Maximum Drop in Temperature
Before coiling, ΔT was independent of the pulse rate at all pressure levels (Fig 8). Within a given pulse rate, ΔT was not influenced by the systemic pressure. Treatment with GDCs largely reduced ΔT at all pulse rates and all systemic pressures (P < .01). At pulse rates of 40 and 80 beats per minute, a major reduction in ΔT was observed with the values for the different pressure levels being concentrated, while the corresponding reduction in ΔT at the pulse rates of 60 and 100 beats per minute was less and dispersed for the various pressure levels (Fig 8).
The maximum drop in temperature, ΔT, created by injections of normal saline at room temperature at various systemic pressures (P) and pulse rates (f), both before (left) and after (right) the insertion of GDCs. Coiling significantly reduced ΔT, which was dependent on the pulse rate but independent of the systemic pressure.
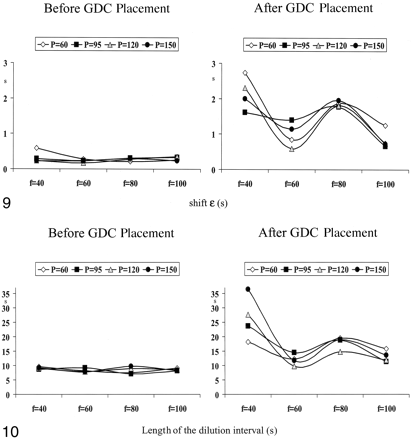
Pressure-Flow Shift
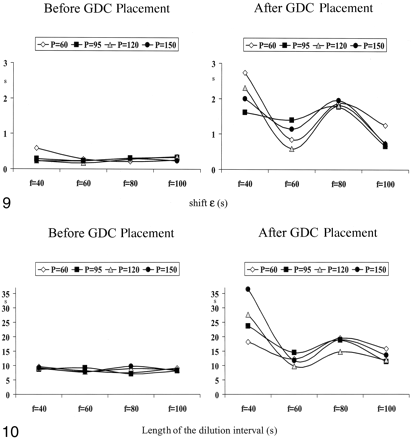
The pressure-flow shift, ε, was slightly higher at the lowest pulse rate (40 beats per minute) and the lowest systemic pressure of 60 mm Hg (P = .02, Fig 9). Apart from this, ε was independentof the pulse rate and pressure before the insertion of GDCs. After treatment with GDCs, a distinct dissociation of pressure and flow (represented by an increase in ε) occurred (Fig 9), with P < .01 at a pulse rate of 40 and 80 beats per minute at all pressures, P < .03 at a pulse rate of 60 beats per minute at all pressures, and P < .05 at a pulse rate of 100 beats per minute. A pressure of 120 mm Hg did not reach statistical significance.
The dissociation of pressure and flow (shift ε) at various systemic pressures (P) and pulse rates (f), both before (left) and after (right) the insertion of GDCs. ε was independent of the pulse rate and pressure before the insertion of GDCs. With the presence of GDCs in the aneurysm, it increased depending on the pulse rate.
Dilution Interval
The length of the dilution interval was not affected by changes in pulse rate or systemic pressure before GDCs were placed into the aneurysm (Fig 10). After the insertion of GDCs, the dilution interval was dramatically prolonged at a pulse rate of 40 beats per minute (P < .01). At this pulse rate, the dilution interval was more prolonged the higher the systemic pressure was (P = .002, Fig 10). At the pulse rates of 60 and 100 beats per minute, the dilution interval was affected to a lower extent by GDC placement.
The length of the dilution interval represented by the total time of change in temperature occurring after injection of 5 mL/s over 4 seconds room temperature normal saline at various systemic pressures (P) and pulse rates (f), both before (left) and after (right) the insertion of GDCs. It was not affected by variations in systemic pressure and pulse rate before GDC placement. There was a marked prolongation of the dilution interval after GDC placement at a pulse rate of 40 beats per minute with a concomitant positive correlation to the systemic pressure.
Synopsis
Before the insertion of GDCs, pressure at the dome of the aneurysm was inversely related to pulse rate and positively correlated to the systemic pressure. The flow at the dome of the aneurysm seemed to be quite constant despite variations in pulse rate and systemic pressure.
The insertion of GDCs into the aneurysm had a moderate effect on the modulation of pressure at the dome of the aneurysm. Flow was reduced markedly, as indicated by flattening of the thermodilution curve. These alterations were best expressed at a pulse rate of 40 beats per minute and were almost independent of the systemic pressure.
Discussion
It has been recognized that hemodynamic forces are closely linked to the genesis, growth, and even rupture of saccular intracranial aneurysms (6). One could assume that the same hemodynamic forces take action when coil compaction occurs. Our findings revealed a clear increase in intra-aneurysmal pressure with increasing systemic pressure, both before and following GDC placement. Although such a pressure increase might not rupture the aneurysm as long as flow stagnation is complete, it could represent a predisposing factor for coil compaction by constantly exerting a pulsating hammer on the mass of coils.
Under this premise, patients in the cold phase after their subarachnoid hemorrhage (SAH) could benefit from rigorous blood pressure control. On the other hand, in the acute phase following SAH, patients may have problems with increased intracranial pressure and even require vasopressor agents to increase their blood pressure and maintain an adequate cerebral perfusion pressure. One of the adverse effects of vasopressor agents is a marked increase in heart rate. However, our findings indicate that a high pulse rate does not seem to be favorable with respect to flow stagnation in a coiled aneurysm. It may be worthwhile to control the heart rate in the neurointensive ward and not merely focus on blood pressure control.
There is also some uncertainty about the appropriateness of heparinization immediately following a coil procedure. In our institution, we may administer heparin as a continuous infusion, because coils may prolapse into the lumen of the parent artery. The argument against this is the concern for reinitiation of flow in the aneurysm, and hence, the risk of rebleeding. As we presently show, flow is considerably attenuated after coil insertion even when there is a total lack of the ability to form a thrombus. Hence, with respect to the coiled aneurysm, it might be safer than we previously believed to treat a patient with SAHfor acute deep venous thrombosis or a fresh lung embolus with heparin.
Hemodynamic versus Hydrodynamic Effect of GDCs
Our main finding is the observation of a marked reduction in intra-aneurysmal flow after treatment with GDCs even without the presence of thrombosis. In other words, GDCs have a hydrodynamic effect. Boecher-Schwarz et al (5) did not find a significant reduction in intra-aneurysmal pressure, flow, or impact after GDC placement in a rabbit ex vivo model. Their in vitro tests were performed by means of perfusion with normal saline as well as heparinized rabbit blood, and they found even less hemodynamic changes with GDC placement when the model was perfused with blood as compared with saline (5). They assigned this discrepancy to a higher “unphysiologic” intra-aneurysmal pressure during perfusion with blood, which increased the aneurysmal wall tension and thereby masked the coil effect (5). Boecher-Schwarz and colleagues (5), however, performed no direct measures of intra-aneurysmal flow and assumed unchanged flow in the aneurysm after GDC placement merely from the lack of difference in hysteresis. In the present study, GDCs markedly reduced flow in the aneurysm both when the aneurysm was perfused with heparinized dog blood and when it was perfused with normal saline. Our findings are in consensus with the observations of Gobin et al (4). They studied flow in silicone aneurysms perfused by 40% glycerol aqueous solution by means of a video camera (4). Partial coiling of the aneurysm resulted in slower and incomplete filling and washout in the aneurysm (4). This finding is parallel to our observations with decreased ΔT (incomplete filling) and prolonged dilution interval (slower washout). Comparing perfusion with blood to perfusion with normal saline, one could anticipate that the difference in viscosity between these two liquid models would result in different flow both before and after the insertion of coils. We did not detect significant differences in flow before coiling when we compared perfusion with blood to perfusion with normal saline (in vivo versus in vitro). Steiger et al (10) studied hemodynamic stress in experimental aneurysms perfused with liquids of varying viscosity. They concluded that the viscoelastic effect of the perfusion solution did not influence intra-aneurysmal flow velocities in bifurcational aneurysms, but did influence the velocity in lateral aneurysms due to generally higher inflow into terminal aneurysms (10). Possibly, there was a relatively high inflow into our lateral aneurysm due to its relatively wide neck. This might explain the quite similar flow in the aneurysm during perfusion with blood as well as normal saline.
The present finding of a marked similarity in pressure and flow dynamics between the experimental canine aneurysm in vivo and in vitro is of great value for further hydrodynamic studies. First, we can delineate hydrodynamic effects without possible confounding effects of thrombosis within the aneurysm or on the pressure-temperature wire. Second, the silicone phantom allows rigorously designed studies at much greater precision than in vivo studies. In the phantom, all parameters can be exactly controlled and repeated to an almost unlimited extent. Apart from being more exact and reproducible as well as cheaper, the perfusion model may spare the life of many laboratory animals, and hence, represents a tool for reduction, refinement, and replacement in accordance with the Guide to Searching for Alternatives to the Use of Laboratory Animals (11). Our silicone vascular model could thereafter be considered superior to those used in other dog experiments to evaluate the influence of changes in systemic pressure and pulse rate on aneurysmal flow and pressure. A latex vascular bench model or similar devices offer the unique opportunity for every neurointerventionalist to try new, hands-on techniques before including them in their therapeutic armamentarium. In a model, the behavior of new materials can be studied both by radiologic means and by direct observation. Personal skills can be acquired without potential harm to patients. Furthermore, in elective cases, one could reconstruct latex models from 3D angiograms of patients and tailor the treatment of that particular patient.
Intra-Aneurysmal Pressure and Flow at Varying Frequencies and Systemic Pressures
Knowledge of the intra-aneurysmal pressure and flow dynamics is essential if one assumes that an aneurysm might rupture when hemodynamic forces exceed the critical point of aneurysm wall stress (12). This might apply not only to untreated aneurysms but also to aneurysms secured by the insertion of GDCs. Hemodynamic stress factors could be decisive in the successful treatment of aneurysms (ie, complete stagnation of flow inside the aneurysm), especially in only partially coiled aneurysms. Particularly in large coiled aneurysms, persistent flow into the aneurysm may lead to coil compaction, and hence, carries the risk of growth or rerupture of the aneurysm. Increased insight into factors that may affect flow into the aneurysm could therefore contribute to an optimization of the perioperative patient care, along with preventative treatment with respect to coil compaction.
In the present study, we observed higher increases in pressure to the saline injections when either the systemic pressure increased or when the pulse rate decreased. To understand this finding, we have to reflect on the physiology of our phantom: The stroke volume was fixed and could not compensate for changes in pressure or pulse rate like the heart may do in a living animal. Hence, increasing systemic pressure flow decreases with augmented resistance. Likewise, when the pulse rate increases, flow increases due to the lack of concomitant reduced stroke volume. Vice versa, a decrease in pulse rate leads to decreased flow. In other words, we observed larger increases in pressure to the saline injections in situations of low flow. Our observation is compatible with the pressure-volume relationship described by Austin et al (6). Studying a model aneurysm wall made of elastic tissue and collagen, they described a nonlinear N-shaped pressure-volume curve; volume increases linearly to increases in pressure until reaching a threshold. Beyond this threshold, further increases in pressure create unproportionally high increases in volume and eventually cause rupture of the aneurysm (6). Increasing the pulse rate caused a curve-shift to the left; that is, toward lower volumes (6).
Before GDCs, the thermodilution curve was insignificantly affected by variations in systemic pressure and pulse rate. Treatment with GDCs thoroughly changed the thermodilution curve by flattening it. This change has been previously described (3). Complete flattening of the curve indicates virtual stagnation of intra-aneurysmal flow, which is the ultimate goal of aneurysm treatment. This effect from the insertion of GDCs was variously efficient, depending mainly on the pulse rate. According to our data, coil treatment was most effective in terms of flow deprivation at a pulse rate of 40 beats per minute. One could assume that the effect from the GDCs was less marked with progressively increasing pulse rate. However, we observed that the flattening of the thermodilution curve was almost equivalent at the pulse rates of 40 and 80 beats per minute. On the other hand, at pulse rates 60 and 100 beats per minute, flattening of the thermodilution curve was equivalently less accentuated. This nodal behavior resembles phenomena described for resonating systems.
Both in experimental and human aneurysms an audible musical bruit has been registered (13, 14). This sound is created by vibrations and was interpreted by Simkins and Stehbens (15) as an indicator of resonating systems. Ferguson (13) states that the incessant vibration of the aneurysmal wall creates a histologic process in the internal elastic membrane similar to the structural fatigue of metals caused by vibration. Bruns et al (16) even showed that wall dilatation of thin-walled, water-filled rubber tubes could be produced by vibration of the water alone in the absence of flow. Such an effect from vibration would be even more enhanced by the presence of self-exciting oscillations; that is, at coinciding resonance frequencies because relatively high strains can be produced by relatively low forces (17). Thus, a defined resonance for each aneurysm might exist and a specific combination of pressure and pulse rate might burst the aneurysm.
Ideally, the mere effect of systemic pressure and pulse rate on intra-aneurysmal flow should be studied under constant flow conditions through the parent artery. Furthermore, it would be of interest to observe possible flow changes in the aneurysm with varying systemic pressure and flow in a similar manner in the parent artery. This is the subject of ongoing studies in our laboratory.
Conclusion
The insertion of GDCs reduces flow in the aneurysm, even in the absence of thrombus, indicating that coils have a hydrodynamic effect per se. This reduction in flow was dependent on the pulse rate. Our observations were compatible with resonance phenomena in the aneurysm.
References
- Received September 19, 2003.
- Accepted after revision November 17, 2003.
- Copyright © American Society of Neuroradiology