Abstract
BACKGROUND AND PURPOSE: Iron oxide–based contrast agents have been investigated as more specific MR imaging agents for central nervous system (CNS) inflammation. Ferumoxtran-10 is a virus-size nanoparticle, taken up by reactive cells, that allows visualization of the phagocytic components of CNS lesions. Ferumoxtran-10 was compared with standard gadolinium-enhanced MR images in this exploratory trial to assess its potential in evaluation of CNS lesions with inflammatory aspects, including lymphoma, multiple sclerosis (MS), acute disseminated encephalomyelitis (ADEM), and vascular lesions.
METHODS: Twenty-three patients with different types of intracranial “inflammatory” lesions underwent standard brain MR with and without gadolinium, followed an average of 10 days later by a ferumoxtran-10 scan. Patients were imaged 24 hours after infusion of 2.6 mg/kg ferumoxtran-10. All MR images were evaluated subjectively by 4 investigators for a difference in enhancement patterns, which could be useful in differential diagnoses.
RESULTS: In 5 cases, (one ADEM, 2 stroke, one cavernous venous vascular malformation, one primary central nervous lymphoma) the ferumoxtran-10 scan showed higher signal intensity, larger area of enhancement, or new enhancing areas compared with gadolinium. Most MS patients showed less enhancement with ferumoxtran-10 than with gadolinium.
CONCLUSION: Ferumoxtran-10 showed different enhancement patterns in a variety of CNS lesions with inflammatory components in comparison to gadolinium. The impact of timing and therapy need further evaluation to better assess ferumoxtran-10 in addition to gadolinium as contrast agents for use in diagnosis and monitoring therapy in patients with CNS inflammatory lesions.
Ultrasmall superparamagnetic iron oxide (USPIO) nanoparticles have been developed as MR imaging agents for the liver and spleen (1) and lymph nodes (2, 3). They have also shown potential for imaging brain tumors (4–6) and macrophage-rich atherosclerotic plaques (7, 8). Use of these nanoparticles could result in an improvement in the imaging of infiltrative disease, in assessing the true extent of disease and providing cellular biologic information relevant to treatment strategies needed for better evaluation of CNS lesions. These virus-size particles markedly enhance the contrast on T1 and T2* MR images and can easily be identified histologically by standard microscopy and by electron microscopy (4, 6). One of these new investigational iron oxide–based MR contrast agents, ferumoxtran-10 (Combidex; Advanced Magnetics, Inc., Cambridge, MA; Sinerem in Europe), showed prolonged and progressive accumulation in intracranial brain tumors, with an enhancement peak at 24 hours after drug infusion that lasts for 4–7 days (4, 5). Histochemical staining for iron in human brain tissue has demonstrated that the MR signal intensity changes are due not to tumor cells, but rather to intracellular endocytosis of the iron oxide particles in reactive cells, mainly at the tumor margin (4–6). This finding led us to investigate the utility of this agent in some other diseases that are associated with reactive cells. We report here our exploratory clinical experience with ferumoxtran-10, which specifically targets reactive astrocytes and other phagocytic cells in CNS lesions (5, 9). Our hypothesis is that ferumoxtran-10, by targeting phagocytic cells, can give additional information that might be beneficial in differential diagnosis and monitoring therapy. In this exploratory trial, we evaluated the use of ferumoxtran-10 in MR imaging in 23 patients with a variety of demyelinating, vascular, and hemopoietic malignant CNS lesions that have inflammatory aspects, with the goal of identifying which lesion type(s) should be investigated in more focused trials.
Methods
This clinical study was conducted in adherence with the Declaration of Helsinki under an investigational drug exemption from the U.S. Food and Drug Administration and received institutional review board approval. Informed consent was obtained from each patient. The investigational drug, ferumoxtran-10, has particles approximately 30 nm in size and consists of iron oxide magnetic crystalline cores that are completely dextran coated (10), which prevents rapid opsonization. It has a plasma half-life of 24–30 hours (5). Previous studies have established that slow infusion of ferumoxtran-10 minimizes adverse reactions (11).
Twenty-three patients (11 women and 12 men; 18–77 years of age; average age, 47 years) with different types of intracranial hematopoietic tumor and/or inflammatory lesions were studied. Diagnoses were based on standard clinical investigations, cerebrospinal fluid (CSF) samples, standard imaging techniques, and, if needed, biopsy, as documented in a patient’s medical history. Multiple sclerosis (MS) was diagnosed on the basis of the Barkhof criteria, CSF studies, and clinical course; acute disseminated encephalomyelitis (ADEM) was diagnosed on the basis of imaging and clinical course; lymphoma diagnosed on the basis of imaging, CSF studies, and biopsy; and stroke and cavernous venous vascular malformation were diagnosed on the basis of both clinical criteria and specific imaging characteristics.
Inclusion requirements were adequate hepatic function; no iron overload; not allergic to iron or dextran drugs; radiologic diagnosis of tumor, cerebrovascular disease, or CNS inflammation. Standard MR imaging of the brain by the usual clinical protocol at our institution or an outside institution in each patient was performed at 1.5T, before and after administration of 0.1 mmol/kg of body weight gadolinium chelate (Omniscan, gadodiamide; Nycomed Imaging AS, Oslo, Norway, or its equivalent). Spin-echo (SE) T1-weighted, fast SE T2-weighted, and proton attenuation (PD) sequences were performed before and (SE) T1-weighted sequence was repeated after gadolinium contrast administration. A ferumoxtran-10 scan was obtained within 30 days of the gadolinium scan; this time window was chosen for logistical and patient care reasons. The dose of 2.6 mg/kg of ferumoxtran-10 was diluted in 100 mL of normal saline and infused intravenously for 30 minutes. Patients were imaged 24 hours after ferumoxtran-10 administration with the same sequences as the unenhanced and gadolinium-enhanced studies. In addition, gradient-recalled-echo (GRE) T2*-weighted and, in some cases, diffusion-weighted imaging (DWI) sequences were performed for the ferumoxtran-10 studies. Perfusion sequences were not performed, because the ferumoxtran-10 can be administered only as a slow infusion, and bolus injection may activate mast cell degranulation. DWI was obtained only in a few cases, and most of the baseline studies did not have DWI, so no comparison was possible. Because this was an exploratory study, images were evaluated qualitatively by consensus between 2 neuroradiologists and 2 neurosurgeons for the intensity and extent of the enhancement of the lesions with gadolinium and with ferumoxtran-10. All 4 evaluators were aware of the clinical status of the patients. The gadolinium-enhanced scans for each patient were compared with the nonenhanced images and subjectively scored as no enhancement, faint enhancement, or good enhancement. The ferumoxtran-10-enhanced study for each patient was then compared with the gadolinium-enhanced study and evaluated as no enhancement, less enhancement than gadolinium (−), equal enhancement to gadolinium (=), increased enhancement to gadolinium (±), or larger/additional enhancement than gadolinium (++). These categories rank visually discernible enhancement differences on the same lesion between the 2 agents. The last category (larger/additional) designated either a significantly larger volume of enhancement or additional lesions not seen on the gadolinium study. T2 and GRE T2 images were not compared in this way, because most of the baseline studies did not have GRE T2*-weighted sequences and, as we reported earlier (4), T2 signal intensity changes were less readily detected.
Results
Twenty-three patients with 8 different types of lesions in 3 broad categories (demyelinating, vascular, and hemopoietic neoplasms) were examined by ferumoxtran-10 MR imaging (Table 1). One patient (no. 10) had 3 different types of lesions (MS, stroke, meningioma). The demyelinating diagnoses were 7 MS and 3 ADEM; vascular lesions were 3 stroke, 3 cavernous venous vascular malformation, and 2 vasculitis; and for hemopoietic neoplasms 5 primary central nervous lymphoma (PCNSL) and one inflammatory myofibroblastic (plasma cell) tumor. Most patients (17/23) were imaged within 15 days of the baseline gadolinium scans, minimizing progression or treatment-related differences. No adverse events were observed due to ferumoxtran-10 administration.
Demographics and MR imaging results with iron particles in patients having different intracranial lesions
The Table summarizes the demographic data, patients’ diagnoses, and data about steroid treatment and compares the enhancement seen with the 2 contrast agents on T1-weighted images. In 5 patients (one ADEM, 2 stroke, one cavernoma, and one PCNSL), the ferumoxtran-10 T1-weighted scan showed more enhancement of lesions than on the gadolinium T1 scan: higher signal intensity or larger area of enhancement or new areas of enhancement. In 5 patients (2 MS, one stroke, one PCNSL, and one cavernous vascular malformation) no enhancement was seen with either gadolinium or ferumoxtran-10. Three MS patients showed faint enhancement with gadolinium and no enhancement with ferumoxtran-10. In the remaining 12 cases, the images showed enhancement for both but less enhancement with ferumoxtran-10 than with gadolinium.
Demyelinating Lesions
In 3 of the 7 patients with MS, ferumoxtran-10-enhanced imaging showed some lesion enhancement, but less intense enhancement than gadolinium. In 2 patients with MS, ferumoxtran-10 did not enhance lesions that gadolinium enhanced. In 2 MS patients, no lesion enhancement was seen with either contrast agent. In one case, the gadolinium enhancement was very intense, but only very faint enhancement with ferumoxtran-10 could be detected (Fig 1). In one (no. 7) of the 3 patients for whom ferumoxtran-10 enhancement was detected on T1-weighted images, the GRE T2* images, but not the FSE T2 images, also showed a markedly low signal intensity focus, consistent with a relatively high concentration ferumoxtran-10. In all of the other MS patients, neither FSE T2 nor GRE T2* images showed changes after ferumoxtran-10 infusion.
Patient 6, with MS. Axial T1-weighted images without (A) and with (B) gadolinium show intense enhancing lesions in the left frontoparietal region. One day later, the lesions showed minimal enhancement with ferumoxtran-10 on axial T1-weighted image (C). The lesions show no significant change on T2 (D) and GRE T2*-weighted (E) images. Notice the low-intensity changes in blood vessels caused by the blood pool agent ferumoxtran-10.
One of the 3 patients with ADEM, shown in Fig 2, showed more enhancement in the brain stem lesion on T1 images with ferumoxtran-10 than with gadolinium. Low-intensity areas corresponding to the same lesions were visible on T2 and GRE T2* images. The lesions in the 2 other ADEM cases enhanced more on gadolinium-enhanced images than on ferumoxtran-10 images. No enhancement was observed with the T2 and GRE T2*-weighted sequences in these patients.
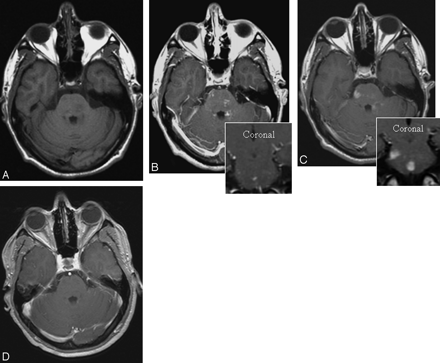
Patient 1, with ADEM. Axial T1-weighted images without (A) and with (B) gadolinium (inset coronal) show faint, subtle enhancement in multiple brain stem lesions. Six days later (C) significant, more prominent, larger ferumoxtran-10 enhancement can be seen on the same site (inset, coronal). Three months later (D) the lesions no longer enhance on T1-weighted images with gadolinium.
In 2 patients (Figs 3 and 4), the clinical diagnoses were uncertain after extensive clinical evaluations and the patients were biopsied to differentiate tumor from demyelinating pathologies. Histopathology revealed demyelinating inflammatory lesions in both patients. In both of these patients, ferumoxtran-10 enhancement was less intense than gadolinium enhancement. These cases suggest that the blood-brain barrier (BBB) may be less permeable to ferumoxtran-10 than gadolinium for demyelinating inflammatory lesions, except in some cases of ADEM.
Patient 2, with biopsy-confirmed ADEM. Axial T1-weighted images without (A) and with (B) gadolinium show multiple, confluent, strongly enhancing lesions around both lateral ventricles. Six days later (C), smaller, less-intense areas were visible with ferumoxtran-10 at the same sites. The lesions show no significant change on T2- and GRE T2*-weighted images (D and E).
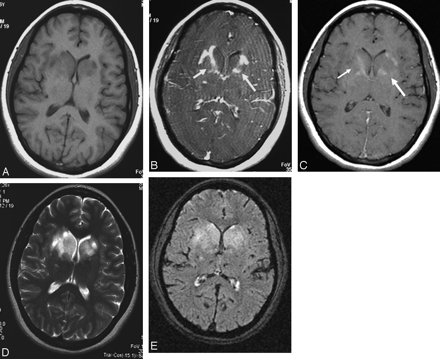
Patient 4, with biopsy-confirmed MS. Axial T1-weighted images without (A and B) and with (C and D) gadolinium show multiple, bilateral temporoparietal, enhancing lesions. Six days later (E and F), smaller, less-intense areas were visible with ferumoxtran-10 at the same site on T1 MR.
Vascular Lesions
Two stroke patients showed more enhancement with ferumoxtran-10 than gadolinium. In one case, there was an intrinsic T1 high intensity visible on the unenhanced images caused by a small hemorrhage, which complicated the evaluation, because iron oxide particles and hemorrhage may show similar signal intensity changes. On GRE T2*-weighted images, a subtle low-signal-intensity area was observed; no change was seen on T2-weighted images. In the other case, the enhancement with ferumoxtran-10 was much more intense than with gadolinium. The time interval (13 days postictus vs 3 days) may have played a role, because parenchymal contrast enhancement with gadolinium usually ensues within 4–7 days after the ictus and can persist for weeks (Fig 5). On GRE T2*-weighted images and T2-weighted images, the lesion showed prominent low-signal-intensity changes consistent with higher concentration iron oxide deposition. The primary diagnosis of the third stroke patient (no. 10) was MS, but she also had small lacunar infarcts and a meningioma. The small vascular lesions did not enhance with either contrast agent, whereas the meningioma did enhance with both agents, but gadolinium enhancement was more prominent.
Patient 11, with stroke. Three days after ictus, axial T1-weighted images without (A) and with (B) gadolinium show no significant enhancement in left insular cortex, basal ganglia, and frontoparietal lobe. The corresponding DWI, T2, and fluid-attenuated inversion recovery images showed high intensity (not shown) consistent with acute middle cerebral artery infarction. T1 images obtained 10 days later with ferumoxtran-10 (C) show significant enhancement in the same region. Ninety days later (D), axial T1-weighted image after gadolinium shows no enhancement. It is clear that ferumoxtran-10 enhancement was intense, but because the BBB opening in stroke generally occurs 4–7 days after the ictus, gadolinium may have also enhanced the lesion if the gadolinium scan had been done a few days later.
One symptomatic cavernous venous vascular malformation did not enhance with gadolinium but did enhance intensely after ferumoxtran-10 administration (Fig 6). Low-signal-intensity areas were detected on T2- and GRE T2*-weighted images, which is consistent with higher-concentration ferumoxtran-10 accumulation. The second of the cavernous vascular malformation cases showed intrinsic T1 high-intensity, which is consistent with blood, and did not show significant intensity changes with either contrast agent. There were low-signal-intensity changes around the lesion on both T2- and GRE T2*-weighted images, which is consistent with hemosiderin deposition, and no significant change after ferumoxtran-10 infusion. The third of the cavernous vascular malformation cases also showed intrinsic T1 high intensity, but also some gadolinium enhancement was visible around the lesion. Ferumoxtran-10 enhancement was less prominent, and evaluation was difficult because of the intrinsic T1 high intensity caused by blood. A classic low-intensity popcorn-like pattern was visible after ferumoxtran-10 administration on T2-weighted images, which is consistent with iron deposition caused by ferumoxtran-10 or blood-degradation products.
Patient 13, with cavernous venous vascular malformation. Sagittal T1-weighted images without (A) and coronal T1-weighted images with (B) gadolinium show no significant enhancement in the left pons lesion. Four days later, prominent ferumoxtran-10 enhancement can be seen in the same lesion on T1 MR (C).
In the 2 vasculitis cases, intense dural gadolinium enhancement was seen, and less-intense ferumoxtran-10 enhancement was noted. In the second case, some parenchymal gadolinium enhancement was also adjacent to the dural lesion, which showed about the same intensity with ferumoxtran-10, but in a somewhat different pattern. No significant changes were seen on T2-weighted images.
Hematopoietic Neoplasms
The 5 patients with PCNSL showed varied ferumoxtran-10 enhancement. Three of 4 lesions (bilateral head of caudate, splenium, and temporal lobe) in one patient showed a larger area and higher contrast intensity on T1-weighted images after ferumoxtran-10 infusion (Fig 7). On T2- and GRE T2*-weighted images, significantly low-intensity areas appeared after ferumoxtran-10 infusion. In the second PCNSL patient, a slightly high T1 intensity lesion precontrast showed no enhancement with either contrast agent. Faint low signal intensity was seen on T2- and GRE T2*-weighted precontrast scans that did not change after ferumoxtran-10 administration. We assume that the low-intensity changes were caused by postbiopsy hemorrhaging. In the third lymphoma case, faint tentorium and left facial nerve enhancement were seen with both contrast agents, with no significant difference in intensity. T2-weighted images did not show a relevant difference in signal intensity.
Patient 18, with PCNSL. Axial T1-weighted images without (A) and with (B) gadolinium show intense ring-enhancing lesions in the head of both caudate nuclei. In the T1-weighted image with ferumoxtran-10 produced 15 days later (C), the lesions in the caudate head are larger and show more intense enhancement. Another lesion is visible in the splenium of the corpus callosum that also shows larger and more intense enhancement than seen on the baseline gadolinium image (not shown). Low-intensity changes in both lesions are visible on T2-weighted images after ferumoxtran-10 administration (D and E).
In the last 2 lymphoma cases, the multiple lesions showed more enhancement with gadolinium and less or no enhancement with ferumoxtran-10. In case 21, T2- and GRE T2*-weighted images had no signs for iron oxide deposition, whereas the last lymphoma case showed faint low-intensity areas consistent with small amount of ferumoxtran-10 deposition.
The plasma cell granuloma (inflammatory myofibroblastic tumor) showed more enhancement with gadolinium than with ferumoxtran-10. Minimal T2 changes were noted postferumoxtran-10, which might be due to hemorrhage because the patient was scanned after biopsy.
Discussion
In this exploratory trial, we evaluated the use of ferumoxtran-10 in MR imaging of 23 patients with a variety of demyelinating, vascular, and hematopoietic malignant CNS lesions that have inflammatory aspects, with the goal of identifying which lesion type(s) should be investigated in more-focused trials.
USPIO nanoparticles have been developed as MR imaging agents for the liver and spleen (1) and lymph nodes (2, 3). They have also shown potential for imaging brain tumor (4–6) and macrophage-rich atherosclerotic plaques (7, 8). These virus-size nanoparticles (Combidex, ferumoxtran-10) markedly enhance the contrast on T1 and T2* MR images and can easily be identified histologically by standard and electron microscopy (4, 6). Ferumoxtran-10 showed prolonged and progressive accumulation in intracranial brain tumors with an enhancement peak at 24 hours after drug infusion, which lasts for 4–7 days (4, 5). Ferumoxtran-10 can increase or decrease the MR signal intensity, depending on both the concentration of drug in tissue and the specific pulse sequences used. Lower concentrations of ferumoxtran-10 on T1-weighted sequences increase signal intensities (12), similar to that seen on gadolinium enhancement; higher concentrations of ferumoxtran-10 can cause profound signal intensity loss also on T1-weighted images (4). Concentration-related signal intensity loss is also detected on T2- and T2*-weighted sequences. Histochemical staining for iron in human brain tissue has demonstrated that the MR signal intensity changes are due not to iron oxide associated with tumor cells, but rather to intracellular endocytosis of the iron oxide particles in phagocytic cells, mainly at the tumor margin (4–6). This finding suggests that other intracranial lesions containing or surrounded by reactive cells may also take up ferumoxtran-10, permitting enhanced detection of the lesion. Agents such as ferumoxtran-10 appears to be selective for areas of phagocytic cells, which may either facilitate the correct diagnosis or be used to monitor therapy.
Whereas the accumulation of some USPIO agents within macrophages is well established, their route of transport to the macrophages in tissues is not yet well defined. Several mechanisms have been suggested: (1) USPIOs are endocytosed by activated blood monocytes that migrate into the pathologic tissues; (2) transcytosis of USPIOs through the endothelium and migration of the USPIOs into the tissue followed by progressive endocytosis of these USPIOs by in situ macrophages; (3) transport of USPIOs into the pathologic tissue, in some cases via the inflammatory neovasculature (vasa vasorum) irrigating the media and adventitia in atherosclerotic lesions (13).
Macrophages play a key role in tissue degeneration, and their visualization can provide direct insights into the MS-related inflammatory activity itself. Recent studies demonstrated that accumulation of macrophages in inflammatory lesions can be visualized in vivo by systemic intravenous administration of ferumoxtran-10 (13). In our MS patients, ferumoxtran-10 showed less or no enhancement compared with the well-established (4, 5, 14–18) gadolinium.
Patients 2 and 3 (Figs 3 and 4) each presented with acute clinical symptoms and enhancing lesions with uncertain diagnosis of tumor versus demyelinating disease after extensive clinical evaluation. Biopsies were taken from both patients, and histopathology revealed demyelinating inflammatory lesions. In both patients, ferumoxtran-10 enhancement was less intense than gadolinium enhancement, which suggests that the BBB may be less permeable to high-molecular-weight ferumoxtran-10 than gadolinium for demyelinating inflammatory lesions, except in some acute demyelination cases (Fig 2). In 3 other demyelinating cases in which the difference between the 2 scans were >7 days (3, 5, 7), timing and/or therapy also could have affected the results. For logistic reasons to accrue patients in an exploratory study and in some cases not to interfere with patient care, we had to allow a time window between the scans, even though timing in inflammatory cases can be an important factor. In a new study, we have narrowed this window to 24 hours, and we perform an additional gadolinium scan after the ferumoxtran-10 scans. Another problem related to the 30-day time window is intervening treatment. Most of the patients had no steroid treatment between the 2 scans, but 5 of the cases have had steroid therapy during this time period and steroid therapy was suspended for one patient between the 2 scans. In 5 cases, no definitive data are available about steroid treatment (Table 1).
In a phase II study (19) of ferumoxtran-10 in 10 MS patients with acute exacerbations, enhancement was reported in 9 patients with ferumoxtran-10 and in 7 with gadolinium. In 2 other MS patients, the lesions enhanced only with ferumoxtran-10, which suggests that the enhancement with ferumoxtran-10 is not superimposed on the enhancement with gadolinium and that the 2 contrast agents complement each other.
These findings are consistent with the theory based on animal studies that gadolinium chelate extravasation and USPIO accumulation are not uniform across CNS sites of acute inflammation and are not always associated with significant BBB disruption (20). In addition, Floris et al suggested that gadolinium enhancement reflecting vascular leakage occurred concomitantly with the onset of neurologic signs and clearly preceded monocyte infiltration (as imaged by a USPIO contrast agent), which was maximal only during full-blown experimental allergic encephalomyelitis (21). These early clinical and animal findings suggest a different mechanism and timing; further study is needed with intravascular ferumoxtran-10 and labeled monocytes, in addition to gadolinium-enhanced scans (22, 23).
In acute stroke, time is a very important factor for contrast enhancement, (24–26) because the BBB opening is biphasic, occurring at 3–6 hours and again beginning at 4–7 days. One of the stroke patients reported here had striking enhancement with ferumoxtran-10 but not gadolinium; however, the different times of MR images with gadolinium (3 days postictus) compared with those for ferumoxtran-10 (13 days postictus) are almost certainly responsible.
Another important issue in stroke is hemorrhage because its degradation products contain iron, which can also affect the MR images. The most critical time after bleeding is the acute phase (1–3 days) for T2 and the subacute phase (4 days to several weeks) for T1, because the T1 and T2 changes caused by hemorrhagic components are similar to the changes seen with the ferumoxtran-10. This problem was carefully evaluated and described in detail in our previous report (5) of preoperative and postoperative serial MR on 7 tumor patients. Calcification and necrosis can also cause intensity changes, but, even with a time window of 30 days, the formation of new calcified or necrotic sites are unlikely, especially because in 16 of 23 patients the time window between gadolinium and ferumoxtran-10 scans was <2 weeks.
In 4 patients with different pathologies (a single case each of ADEM, stroke, cavernous venous vascular malformation, and PCNSL), the ferumoxtran-10 scan showed higher signal intensity and/or larger area of enhancement and/or new enhancing area(s) compared with gadolinium, most likely not related to the timing between scans. In some cases, the difference between MR enhancement with the 2 different contrast agents (gadolinium vs ferumoxtran-10) were very obvious and appear to be due to different pharmacology and pathophysiology. Gadolinium has a short plasma half-life of about 30 minutes, and ferumoxtran-10 has a long half-life of 24–30 hours. BBB leakage is essential for contrast enhancement with both contrast agents, but the degree of the opening of the barrier may be an important differential factor (17, 18). Ferumoxtran-10 is the size of a small virus, and gadolinium-based contrast agent is a small molecule chelate of gadolinium that may be able to cross the BBB more effectively because of its small size. Another important difference between gadolinium and ferumoxtran-10 is that ferumoxtran-10 is endocytosed by phagocytic cells (macrophages, glial cells; 4, 5, 10), unlike gadolinium, which does not enter cells. This cell-specific uptake difference may allow lesion enhancement even with a small area of BBB leakage if the BBB opening permits iron oxide particles to slowly cross, because of the long plasma half-life of feromoxtran-10.
In short, ferumoxtran-10 is a high-molecular-weight, phagocytic cell–specific, intracellular contrast agent with a very long plasma half-life, whereas gadolinium is a low-molecular-weight, extracellular, interstitial contrast agent with a short plasma half-life. If these differences can be exploited to find and evaluate those lesions that recognize the differences, our ability to differentially diagnose and follow patient therapy may improve.
More patients are needed to resolve these issues and determine when ferumoxtran-10 imaging will add useful clinical information to that obtained with gadolinium for differential diagnosis or treatment response of patients presenting with MS and other inflammatory cells containing CNS lesions. It has been shown that the BBB breach allowing gadolinium enhancement and the phagocytic cell invasion are not simultaneous events (13, 20, 21), but the exact timing of these pathophysiologic changes and their role in disease are still not clear, especially in different forms of MS. In addition, the gadolinium scans should be performed more closely in time, both before and after the ferumoxtran-10 scans to better determine what differences are due to the different contrast agents and what are due to timing issues.
Three promising observations may benefit patients presenting with inflammatory cell–mediated disease. First, iron oxide–based contrast agents, such as ferumoxtran-10, label cells for as long as 7 days and can be visualized also on low-magnetic-field (0.15T) intraoperative MR (22). This allows time for in vivo MR imaging and, if biopsy is indicated, correlating pre- and postoperative images with pathologic findings by localizing the labeled inflammatory cells with iron stains (27). Second, peripheral mononuclear cells can be labeled with an SPIO agent, a different iron oxide particle (ferumoxide) and the “cellular contrast agent” can then be tracked by MR and histology entering CNS inflammatory lesions (22, 23). Therefore, both ferumoxide-labeled mononuclear cells versus ferumoxtran-10 and gadolinium contrast agents can be tracked crossing the BBB into CNS inflammatory lesions. Indeed, the first use of iron-labeled cells in human brain has recently been reported (28). Third, other investigators have shown that the use of high-magnetic-field MR systems to make quantitative measurements of the first-pass gadolinium enhancement of the tissue 1H2O longitudinal relaxation rate constant (R1) can detect quite subtle changes of BBB properties (29–31).
Our hope in the future is that we can use all these different contrast agents and BBB imaging techniques together to find out more about the BBB, provide disease-specific information, and monitor therapy in CNS lesions with an inflammatory component.
Conclusion
MR imaging with ferumoxtran-10 shows different size and locations of lesions in PCNSL and other CNS inflammatory lesions than imaging with gadolinium. In some cases, ferumoxtran-10—in other cases, gadolinium—showed more intense or different volumes of enhancement. These differences presumably are related to the molecular size, phagocytic cell specificity, and/or the plasma half-life differences of the 2 agents. Ferumoxtran-10 did not enhance as well in MS as it did in other inflammatory lesions or tumors, which may be indicative of different BBB defects in MS, PCNSL, and stroke. Differential diagnosis might be improved by exploiting the observation that most MS lesions did not enhance with ferumoxtran-10, whereas in some cases lymphomas and strokes enhanced equivalently or better. Timing and treatment factors significantly influence the BBB in these lesions and may in part explain the differences. Further studies that control for these factors are necessary to establish the utility of ferumoxtran-10 compared with gadolinium-based contrast agents, for use in both diagnosis and monitoring therapy in intracranial inflammatory lesions.
Acknowledgments
This project was funded by grant NS034608 from the NINDS, PHS Grant 5M01 RR000334, and by the Portland VA Medical Center. Ferumoxtran-10 (Combidex) was provided by Advanced Magnetics, Cambridge, MA. Our thanks to Ruth Whitham, MD, Jesus Lovera, MD, and Dennis Bourdette, MD, for helping with the MS and ADEM cases; and to Marianne Haluska, ANP, Lisa Bennett, and Shannan Walker-Rosenfeld for technical assistance.
Footnotes
This project was funded by grant NS034608 from the NINDS, Public Health Service grant 5 M01 RR000334, and the Portland VA Medical Center. Ferumoxtran-10 (Combidex) was provided by Advanced Magnetics, Cambridge, MA.
Part of this study was presented as “Ferumoxtran-10 and Gadolinium as Imaging Agents in PCNSL and Other CNS Inflammatory Lesions” at the 42nd annual meeting of the American Society for Neuroradiology, June 5–11, 2004, Seattle, Washington.
References
- Received January 31, 2005.
- Accepted after revision April 19, 2005.
- Copyright © American Society of Neuroradiology