Abstract
INTRODUCTION: Diffusion-weighted (DW) MR imaging enables early identification of ischemic lesions in stroke. Stroke subtype may be related to different lesion patterns. The aim of this study was to analyze the subtype of ischemic lesions as determined by the Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria by using DW MR imaging.
METHODS AND RESULTS: In this study, 510 consecutive patients with ischemic stroke (95%) and transient ischemic attack (5%) aged 65 ± 12 years were investigated by use of DW MR imaging within 48 hours of the clinical onset of symptoms. Lesions on DW imaging were classified as single, scattered, or multiple lesions in one vascular territory and multiple in more than one vascular territory. We found a significant overall association of DW imaging lesion patterns and classification with stroke subtype by using the TOAST criteria (P < .001). Single corticosubcortical lesions (P < .01) and multiple bilateral lesions in the anterior (AC) and posterior circulation (P < .001) on DW imaging were significantly associated with a cardiac embolic source. Multiple unilateral lesions in the AC were significantly associated with large-artery arteriosclerosis. Because of the 15-mm criterion for small-artery occlusion, cryptogenic stroke was significantly associated with subcortical lesions ≥15 mm.
CONCLUSION: We found a strong relationship between stroke subtype and DW imaging lesion pattern. The finding of multiple bilateral lesions was significantly associated with a cardiac embolic source, which may be caused by a specific thrombus texture with a tendency for embolus dissemination.
The precise diagnosis of stroke subtype is important for further management, including diagnostic and therapeutic decisions, that may be influenced by the prognosis and recurrence rate of the underlying etiology. The Trial of Org 10172 in Acute Stroke Treatment (TOAST) subtype classification is the most widely used and is based on clinical findings, imaging results, and further diagnostic workup.1 Diffusion-weighted (DW) MR imaging already has a substantial impact on early stroke diagnosis and therapy. In contrast to CT and conventional MR imaging without diffusion weighting, DW imaging provides detection of lesions in the first hours after the onset of clinical symptoms.2 Furthermore, DW imaging is superior in detecting very small ischemic lesions due to the high signal- intensity-to-noise ratio and has the capacity of differentiating between chronic and acute lesions.2 Clinically silent, small lesions may influence the diagnosis of stroke subtype in ischemic stroke when multiple lesions are detected on DW imaging.3 The presence of multiple ischemic lesions suggests embolism from the heart or the aortic arch or stenosis of one of the extra- or intracranial large arteries if confined to one vascular territory. Multiple infarcts in more than one vascular territory, especially bilateral lesions, strongly argue for a proximal source or systemic cause.4 Patients with ischemic lesions due to multiple emboli may be at a higher risk of stroke recurrence in the acute phase and therefore may benefit from a specific therapy—for example, anticoagulation.
Thus, lesion patterns may be closely associated with stroke etiology and may have important clinical implications. We therefore investigated the association of the stroke subtype defined by TOAST criteria1 in a large population of stroke patients.
Patients and Methods
All patients with acute onset of a focal neurologic deficit admitted to our neurologic emergency unit, where we see an average of about 2000 patients per year, were included in the study. Patients with neurologic symptoms due to an etiology other than cerebral ischemia (eg, cerebral hemorrhage, tumor, Todd’s palsy) and patients with contraindications for MR imaging where excluded. Of the patients receiving DW MR imaging, all 510 with acute hyperintense lesions were included in this study. All patients received extra- and transcranial color-coded duplex sonographic examination, transthoracic echocardiography, and 24-hour electrocardiographic monitoring as well as blood and urine laboratory tests. Additional transesophageal echocardiography was performed in 73% of all patients, in whom transthoracic echocardiography, holter electrocardiography, and duplex sonography of the extra- and intracranial vessels did not show evidence for cardiogenic or large-artery embolism. In all cases, stroke risk factors were recorded.
MR Imaging
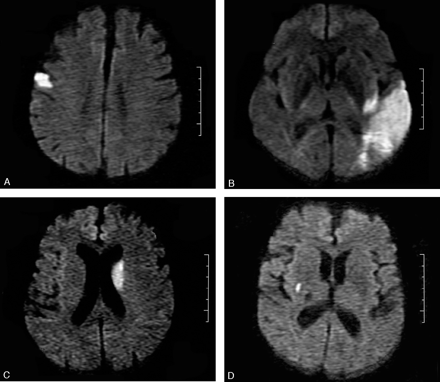
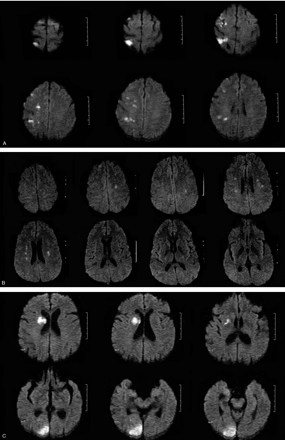
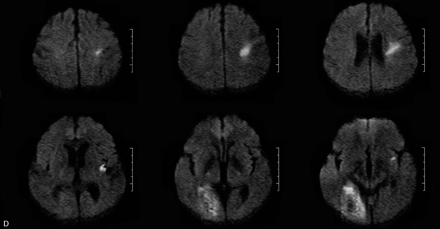
MR imaging was performed within 48 hours of symptom onset by using a 1.5T whole-body scanner (GE Medical, Milwaukee, Wisc) equipped with echo-planar imaging data capability designed to obtain rapid diffusion images. The study protocol included a T2-weighted gradient spin-echo axial sequence (acquisition time, 58 seconds; repetition time, 4000 ms; echo time, 95 ms; 19 sections; section thickness, 6.0 mm: matrix, 256 × 256; echoes per excitation, 3) to detect old and potentially new ischemic lesions, hemorrhage, and mass lesions, and a diffusion-weighted sequence (acquisition time, 68 seconds; repetition time, 260 ms; echo time, 184 ms; 20 sections; section thickness, 6.0 mm; matrix, 128 × 128) to detect recent ischemic lesions. The diffusion gradients were applied along the x, y, and z axes, thus minimizing anisotropic effects, and the DW imaging sequence was acquired with 3 different b values (b = 0, 500, and 1000 s/mm2). A positive DW imaging scan was defined as high signal intensity on the b1000 image. The apparent diffusion coefficients (ADCs) were calculated for each pixel and composed in an ADC map. In all cases with a lesion on DW imaging, we also reviewed the ADC map and noted whether high-signal-intensity areas on the b1000 image showed low, high, or normal signal intensity when comparing the affected area to the corresponding contralateral area. All MR images were assessed by both a neuroradiologist and a neurologist blinded to the clinical findings. Ischemic DW imaging lesions were classified as (1) single lesions (corticosubcortical, cortical, subcortical; Fig 1A–C) or (2) scattered lesions in one vascular territory (anterior [AC] or posterior [PC] circulation), and (3) multiple lesions in more than one vascular territory (unilateral AC, PC circulation, bilateral AC, unilateral AC and PC, bilateral AC and PC; Fig 2A–D). Multiple lesions were defined as in previous studies as not connected hyperintense DW imaging lesions.3–5
A, Single cortical lesion. B, Corticosubcortical lesion. C, Large subcortical stroke (>15 mm). D, Small subcortical lesion.
A, Multiple lesions in the AC. B, Multiple, bilateral lesions in both ACs. C, Multiple, unilateral lesions in the AC and PC. D, Multiple, bilateral lesions in the AC and PC.
All lesions were allocated to a vascular territory: anterior cerebral artery, middle cerebral artery (proximal, distal and lenticulostriate), posterior cerebral artery, watershed, basilar, and cerebellar arteries, as suggested by Tatu et al.5
Stroke Subtype
Stroke subtype was determined by the TOAST classification criteria1: (1) large-artery atherosclerosis (LAA), (2) cardioembolism (CE), (3) small-artery occlusion (SAO), (4) cryptogenic stroke (undetermined cause), (5) stroke of other determined etiology, (6) 2 or more competing causes.
Diagnosis of stroke subtype was based on clinical findings, cerebrovascular sonography, MR or CT angiography, and cardiovascular examination. Stroke subtype classification was performed by 2 neurologists blinded to the DW imaging lesion patterns other than the lesion size of <15 mm of subcortical infarction for the classification as SAO. The presence of a clinical lacunar syndrome was diagnosed according to the method of Fisher.6
Statistical Analysis
All numerical variables were expressed as mean ± SD. The Fisher exact test was used to analyze the association of DW imaging pattern and stroke subtype. The association of all 12 lesion patterns and TOAST classification was analyzed by Pearson χ2 test with Monte Carlo estimation of exact P values. A level of P < .05 was considered to be significant.
To further analyze the relevance of DW imaging findings for determination of etiology in the cryptogenic stroke subgroup, we calculated the predictive value of DW imaging lesion in patients with cryptogenic stroke for cardiogenic embolism under the assumption that multiple lesions in different vascular territories are highly specific for cardiogenic embolism. To address the question of whether risk factors for SAO or typical white matter lesions are also present in the group with subcortical lesions ≥15 mm, we calculated the incidence of risk factors for SAO such as hypertension, hyperlipidemia, and diabetes in this group.
For statistical analysis, the SAS software package version 8.2 (SAS Institute, Cary, NC) was used.
Results
In this study, 510 consecutive patients with at least one acute, hyperintense lesion on DW MR imaging (301 [59%] men; 209 [41%] women; mean age, 65 ± 12 years) were included. Twenty-four of these patients (5%) had a complete clinical recovery within 24 hours, fulfilling the clinical criteria of a transient ischemic attack.
A single lesion was observed in 302 (59%) patients, scattered lesions in one vascular territory in 38 (7%) patients, and multiple lesions in multiple territories in 170 (33%) patients.
The most common underlying stroke subtype, in 131 (25%) patients, was CE, SAO in 124 (24%) patients, LAA in 117 (23%) patients, undetermined cause (cryptogenic stroke) in 111 (22%) patients, 2 or more competing causes in 17 (3%) patients, and other etiologies in 10 (2%) patients.
The lesion patterns among the different stroke subtypes are shown in the Table. We found a significant overall association between stroke subtype and DW imaging lesion patterns (P < .001).
Imaging characteristics: correlation of lesion patterns with stroke subtype
The finding of a single corticosubcortical lesion was significantly associated with CE (43% [22/51] vs 24% [109/459]; P < .01) and related to LAA (35% [18/51] vs 21% [99/459]; P < .05). Patients with a single, subcortical lesion ≥15 mm were classified as having a cryptogenic stroke (50% [27/54]) due to the 15-mm TOAST criterion1 for SAO; however, a classic lacunar syndrome was seen in 16 (59%) of these 27 patients. Cardioembolic stroke (28% [15/54]) and LAA (19% [10/54]) were not significantly associated with large subcortical stroke. Although small subcortical lesions were only significantly associated with SAO (80% [124/155]; P < .001), in 9 (6%) patients CE and in 16 (10%) patients LAA were found as underlying etiology.
Scattered lesions in one vascular territory were not found to be associated with a specific stroke subtype. Multiple lesions in the unilateral AC were significantly linked to LAA (46% [39/85] vs 18% [78/425]; P < .001) and multiple lesions in the bilateral AC and PC to CE (69% [9/13] vs 24% [122/497]; P < .001).
If LAA was excluded by MR angiography and duplex sonography, we postulated that bilateral infarction in both AC and PC territory or unilateral infarction in AC or PC, respectively, in patients without LAA are specific for CE.
On the basis of these assumptions, the positive predictive value for bilateral DW imaging lesions or unilateral lesions in both AC and PC territory for a cardiogenic cause was calculated as 0.76 (range, 0.54–0.90).
We identified 27 patients with cryptogenic stroke and subcortical lesions ≥15 mm. Of them, 93% had at least one risk factor (hypertension, hyperlipidemia, diabetes) for SAO. Because of the high incidence of risk factors for SAO, however, these findings were not specific for patients with cryptogenic stroke, because the presence of at least one risk factor for SAO was also frequently found in the CE group (124 [95%]), in the LAA group (111 [95%]), and in the group with more than 2 etiologies (17 [100%]).
Discussion
We found an overall association of acute DW imaging lesion patterns with the TOAST stroke subclassification.1 Corticosubcortical lesions were associated with CE and LAA. Multiple lesions in the AC were linked to LAA. We found multiple lesions in bilateral AC and PC to be associated with CE. Therefore, our study confirms in a larger sample the findings of previous studies4, 7 Our results suggest that acute DW imaging lesions may offer information about stroke etiology. A previous study by Kang et al7 investigated the association of DW imaging patterns and TOAST subtype in a smaller population (172 patients) and found similar results. They also observed a higher rate of large corticosubcortical lesions in cardiogenic stroke, but no significant association between LAA and corticosubcortical lesions. The findings in our series resulted from a relatively large number of 10 patients with internal carotid artery occlusions without evidence for embolic origin on cardiac workup. Nevertheless, our study also supports the concept that emboli from cardiac origin lead to larger lesions.
In our series, 24% (27/111) of the patients were classified as having cryptogenic stroke due to the 15-mm criterion of large subcortical infarction. Because classic risk factors were present in 25 of these 27 patients, we cannot argue against a revised classification with a 20-mm cutoff size for classification of SAO as suggested elsewhere.7, 8 By contrast, because a large proportion of patients presented with at least one risk factor regardless of TOAST classification, the finding of risk factors for small-vessel disease lacks of specificity. For the evaluation of scattered and multiple lesions, we applied the classification suggested by Roh et al4 because our aim was the association to the underlying etiology. Some authors regarded the complete middle cerebral artery territory as a whole territory,9 whereas others addressed any scattered lesions as multiple lesions.10
In our study, multiple lesions detected on DW imaging represented more than 30% (170/510) of ischemic strokes, which is a higher percentage than Bogousslavsky et al found11 but similar to the findings of other recent studies4, 7 Our results indicate that multiple lesions in the AC are caused by atherosclerotic disease (LAA). This finding is supported by the results of Bogousslavsky,12 who found a high prevalence (75%) of internal carotid artery stenosis or occlusion in patients with multiple infarcts in one hemisphere. In a consecutive study, Bogousslavsky et al reported that large-artery disease and CE explain approximately 60% of acute multiple infarctions in the unilateral AC.11 These findings are similar to our results.
In our series, cardiogenic embolism was the main cause of multiple bilateral lesions, which is supported by the results of other investigators.3, 7 Nearly one third of our patients with multiple lesions in the AC had bilateral infarcts. The break-up of an embolus arising from the heart or aortic arch may cause multiple lesions; however, the time course of the occurrence of multiple lesions in different vascular territories occurring simultaneously needs further clarification and may result from embolic showers or be due to recurrent emboli. Other possible explanations are diffuse thrombotic or inflammatory processes that lead to multiple small-vessel occlusions. In a number of studies, the detection rates of embolic sources in patients with multiple DW imaging lesions varies.3, 13, 14 The association of a specific pattern of multiple lesions associated with a specific embolic source was described by Kang et al7 in a smaller series and was confirmed by our results.
Our large sample size enabled analysis of the group of patients with cryptogenic stroke separately. Under the condition that LAA can be excluded by noninvasive vessel examinations, a high positive predictive value for unilateral infarction in both anterior and posterior territory or bilateral infarction was found, which raised the question of whether a determination of stroke etiology by using DW imaging can reduce the number of patients for whom a definite etiology cannot be determined. In our series, this was true for 55 (11%) of all patients, for whom scattered lesion patterns or multiple lesions were observed and no embolic source was detected.
Conclusion
In summary, we were able to identify different DW imaging patterns associated with specific stroke causes. With early identification of DW imaging patterns a consideration to the stroke cause may be provided. The finding of multiple lesions in one AC suggests LAA and multiple bilateral lesion cardiogenic embolism.
References
- Received March 17, 2005.
- Accepted after revision June 9, 2005.
- Copyright © American Society of Neuroradiology