Abstract
BACKGROUND AND PURPOSE: A recent trial shows an 8.3 per 100-patient-years' ischemic stroke rate in the territory of the intracranial stenotic artery, despite aspirin treatment. Our aim was to prospectively study the feasibility and outcome of a new intracranial balloon-expandable Apollo stent for symptomatic atherosclerotic intracranial stenosis (SAIS).
MATERIALS AND METHODS: Forty-six patients (41 men and 5 women; median, 54 years of age) with forty-eight ≥50% SAISs were enrolled. Procedural feasibility was evaluated by stent success (residual stenosis ≤30%) and procedural time. The primary end point was ischemic stroke in the target-lesion artery territory, including any stroke and death within 30 days.
RESULTS: Forty-four lesions (91.7%) obtained stent success within a median procedural time of 50.6 minutes. Severe tortuosity correlated with stent failure. Three patients (6.5%, 3/46) had minor strokes within 30 days. All patients were available for follow-up (46 had 30-day follow-up, 45 had 6-month follow-up, 44 had 12- and 18-month follow-up, and 24 had follow-up of ≥24 months), which varied from 1 month to 30.7 months (median, 23.9 months). After 30 days, 1 patient (2.2%, 1/46) developed minor stroke in the target-lesion artery territory at 6.7 months. Primary end point rate was 4.3 per 100 patient years. Angiographic follow-up was performed in 25 patients. Seven restenoses (28%, 7/25) were detected, 1 of which was symptomatic.
CONCLUSION: Angioplasty with the Apollo stent for symptomatic atherosclerotic intracranial stenosis is feasible. Severe tortuosity is an independent predictor of stent failure. Our clinical outcome seems to compare favorably with results of aspirin therapy, but the restenotic rate was high.
Symptomatic atherosclerotic intracranial stenosis (SAIS) is an important cause of ischemic stroke.1–5 Recently, the Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) trial2 recommended that aspirin should be used in preference to warfarin for patients with this disease, though there is an 8.3 per 100-patient-years' ischemic stroke rate in the stenotic artery territory in patients treated with aspirin. Angioplasty or stent placement for SAIS may be a promising option, but it remains investigational.6,7 Most knowledge about stent placement for intracranial stenosis is from case series in which the use of coronary stents was attempted in intracranial vasculatures.8–13 Stent placement seems superior to balloon angioplasty alone in improving posttreatment results and reducing dissection and recoil frequencies.6–16 To date, there have been only 2 pilot studies to respectively evaluate the feasibility of the Neurolink Stent (Boston Scientific, Natick, Mass) or the Wingspan Stent (Boston Scientific), both of which are designed specifically for intracranial stenosis,15,16 and articles about long-term outcome after stent placement of intracranial stenosis are few.13 The goal of this study (ASSIST) was to prospectively evaluate the feasibility and outcome of angioplasty with a new intracranial balloon-expandable Apollo stent (MicroPort Medical [Shanghai], Shanghai, China) for SIAS.
Patients and Methods
Between December 10, 2003, and December 29, 2004, 46 patients with 48 SAISs of ≥50% were enrolled into this study. The institutional ethics committee approved the protocol of this prospective, nonrandomized, and single-center pilot study. Each patient provided written informed consent.
Apollo Stent System
The Apollo stent system, designed specifically for intracranial stenosis, comprises a semicompliant balloon, a stent, and a delivery catheter. The system is delivered over a 0.014-inch microwire. Its maximal crossing profile is 0.9 mm. The stent (0.1-mm stent-strut thickness and 6 cells for each ring) is made of 316L stainless steel with diameters of 2.0–4.0 mm and lengths of 8 and 13 mm. A shorter length (<1 mm) of each ring and only 2 U-shaped links to connect contiguous rings (Fig 1) render the stent flexible enough to negotiate tortuous intracranial arteries. A relatively low pressure (6 atm) to release the stent reduces the likelihood of vessel dissection or rupture. Moreover, a longer distal pliable rapid-exchange portion (30 cm) ensures that the proximal stiff shaft of the delivery catheter will never enter the tortuous segment of the intracranial internal carotid artery or distal cervical vertebral artery.
The Apollo stent.
Inclusion and Exclusion Criteria
Inclusion criteria were as follows: transient ischemic attack (TIA) or minor stroke that occurred within 90 days before stent placement and that was attributed to an angiographically verified ≥50% stenosis of a major intracranial artery; lesion length <20 mm and normal arterial diameter adjacent to the stenosis between 2.0 and 4.0 mm; patient's National Institutes of Health Stroke Scale (NIHSS) score <9; 18–75 years of age; and patient having at least 1 atherosclerotic risk factor (arterial hypertension, diabetes mellitus, hyperlipidemia, hyperhomocysteinemia, and cigarette smoking). Exclusion criteria included nonatherosclerotic stenosis; intracranial hemorrhage in the territory of the stenotic artery within 6 weeks; potential source of cardiac embolism; concurrent intracranial tumor, aneurysm, and cerebral arteriovenous malformation; tandem ≥50% stenosis of extracranial carotid or vertebral artery; known contraindication to heparin, aspirin, clopidogrel, anesthesia, and contrast media; hemoglobin level <10 g/dL; platelet count <100,000; international normalized ratio >1.5 (irreversible) and uncorrectable bleeding diathesis; and life expectancy <1 year because of other medical conditions.
Feasibility Evaluation
Procedural feasibility was evaluated by stent success and procedural time. Stent success was defined as the stent completely covering the lesion, resulting in a ≤30% residual stenosis with good anterograde blood flow. Procedural time was defined as the time between the first angiography and last one during the operation. On the basis of our experience, lesion site (anterior versus posterior circulation), degree (≥70% versus 50%–69%), location (bifurcation versus nonbifurcation), length (>10 versus ≤10 mm), and access between guiding catheter and stenosis (severe tortuosity versus mild and moderate tortuosity) might be 5 important factors relevant to the risk of stent failure or any stroke and death within 30 days. These factors should be validated.
End Point Assessment
The primary end point was ischemic stroke in the target-lesion artery territory including any stroke and death within 30 days. Secondary end points included ischemic stroke in the non-target-lesion artery territory, death from other vascular causes, emergency cerebral revascularization (ECER), and other major hemorrhages. Ischemic stroke was defined as a new focal neurologic deficit of sudden onset that lasted at least 24 hours and that was not caused by hemorrhage on brain CT, which was divided into major (NIHSS ≥9) and minor (NIHSS <9).5,17,18 Ischemic stroke was considered to be definite in the target-lesion artery territory when the neurologic signs correlated with a new infarct on CT in the territory of that artery and probable in the target-lesion artery territory when the neurologic signs were localized to the territory of that artery, but without a new infarct on CT.2 Intracranial hemorrhage (ICH) was defined as evidence of brain parenchyma or subarachnoid space blood on CT. Death from other vascular causes was defined as sudden death or death within 30 days from an acute ischemic or hemorrhagic disease other than ischemic stroke and ICH, such as myocardial infarction or extracranial vascular rupture. ECER was defined as an emergency therapy by intravenous thrombolysis or endovascular intervention for an angiographically verified acute occlusion of a cerebral artery that immediately led to complete patency and entire disappearance of a new focal neurologic deficit within 24 hours, which meets the clinically diagnostic criteria of TIA.5,18 Other major hemorrhage was defined as hemorrhage (other than ICH) requiring hospitalization, blood transfusion, or surgery.
Preprocedural Preparation
MR imaging and cerebral angiography were completed 1–7 days before stent placement. Stenosis was measured manually and blindly by a neuroradiologist (X.-T.X.), according to the WASID trial method.19 Patients were pretreated with 300-mg aspirin plus 75-mg clopidogrel daily for at least 7 days before the operation. Aspirin, 300 mg per day, was continued for the entire follow-up, and clopidogrel, 75 mg per day, for at least 6 months after stent placement. Probucol, an antioxidation agent that can reduce restenosis after coronary angioplasty,20,21 500 mg twice daily, was started 3 or more days before stent placement and was continued for 6 months or more after stent placement. Atherosclerotic risk factors were managed by 1 experienced stroke neurologist (K.-H.D) according to American Heart Association guidelines.22
Stent-Placement Procedure
The procedure was performed by 1 of 2 experienced interventional neuroradiologists (W.-J.J. and B.D.). On the day of the operation, intravenous infusion of nimodipine (0.6 mg/h) was started 2 hours before the procedure to prevent vasospasm. In our previous study, a high intraoperative heparin regimen (a bolus of 3000 U, followed by 800 U, to maintain an activated clotting time between 250 and 300 seconds) did not reduce the risk of target-lesion thrombosis but had a higher ICH risk, in comparison with a low-heparin regimen (a bolus of 2000 U, followed by 500 U/h, to maintain an activated clotting time between 160 and 220 seconds).23 Thus, the low-dose heparin regimen was administrated intravenously in this study. The operation was performed with the patient under local anesthesia. A 6F guiding catheter was delivered to the distal cervical internal carotid or vertebral artery through a 6F sheath in the femoral artery. Under roadmap guidance, a 0.014-inch microwire was carefully steered through the target lesion. The stent system was then advanced to the lesion over the microwire without a balloon predilation. The stent diameter was selected to be the same as the diameter of the normal adjacent vessel (on either side of the stenosis, whichever was smaller) or slightly smaller (1:1 or 0.9:1). The stent length was 1–2 mm longer than the target lesion on both sides. The stent was released by gradual balloon inflation up to 6 atm within 10–20 seconds. Second stents were used when the lesion length was ≥12 mm or the lesion was not completely covered by the 1st stent. After obtaining stent success, the neuroradiologist removed the balloon catheter and left the microwire in the original site for a 10-minute observation. The last angiography was then performed to confirm the patent stented vessel, followed by removal of microwire and guiding catheter.
Postprocedural Management
The patient's blood pressure was controlled within 100–120/60–80 mm Hg to reduce hyperperfusion syndrome, immediately after stent placement through titration of nimodipine or urapidil hydrochloride. Brain CT was performed immediately to exclude ICH and was repeated after 24 hours if the patient had a new neurologic deficit. Heparin was temporarily stopped 3 hours after stent placement to allow removal of the arterial sheath in another 3 hours, and the patient was then switched to subcutaneous low-molecular-weight heparin (Fraxiparine) 0.4–0.6 mL (based on the patient's body weight) every 12 hours for 3 days. Vitals signs and neurologic status were closely monitored throughout the perioperative period.
Follow-Up Study
Patients were scheduled to be followed up until June 2006. Clinical follow-up and assessment of end point events were completed by 1 stroke neurologist (K.-H.D.). Clinical visits were done every day until discharge and at day 30. Clinical follow-up after 30 days was scheduled at an interval of 3 months till 12 months, then every 6 months by clinic visit or telephone. If a stroke was suspected, patients underwent brain CT.
Angiographic follow-up was scheduled after 6 months on a voluntary basis by the patient or clinically when restenosis was suspected. Restenosis was defined as a ≥50% stenosis within the stent or at the edge of stent on follow-up angiography.
Statistics
All data were analyzed according to the intention-to-treat principle. Fisher exact tests were used to assess associations of the 5 angiographic characteristics mentioned previously with the risk of stent failure or any stroke and death within 30 days. Stratification analysis was used to assess significant relationship between the potential risk factor and stent failure, when there was the confounding effect of another factor. Cumulative probability of ischemic strokes, including any stroke and death within 30 days, over time was estimated by the product-limit method. Data pertaining to patients lost to follow-up were censored on the last contact date. All reported probability values were 2-sided, and P values < .05 were considered significant.
Results
This study enrolled 41 male and 5 female patients, with a total of forty-eight ≥50% SAISs. Their median age was 54 years (range, 38–74 years of age). All patients had 1 or more atherosclerotic risk factors, which included hyperlipidemia (87.0%, 40/46), hypertension (76.1%, 35/46), cigarette smoking (60.9%, 28/46), diabetes mellitus (23.9%, 11/46), and hyperhomocysteinemia (17.4%, 8/46). Six patients (13.0%, 6/46) had prior stroke history. The median time from qualifying TIA event (n = 31) or stroke event (n = 15) to stent placement was 19.5 days (range, 2–66 days). Of 46 patients, only 3 patients who had TIA as a qualifying event were treated by stent placement within 7 days, and the other 43 were treated after 7 days. Forty-four patients had a single target lesion, and 2 patients had 2 stenoses (right and left middle cerebral artery stenoses in 1 patient and left middle cerebral artery stenosis and right intracranial vertebral artery stenosis in the remaining one). The angiographic characteristics of 48 stenoses are shown in the Table.
Univariate analyses of characteristics of stenosis hypothesized to be associated with stent failure, and any stroke and death within 30 days
Procedural time for each lesion was 56 minutes ± SD of 24.3 (median, 50.6 minutes; range, 19–125 minutes). Forty-two of 46 patients obtained stent success, including 1 patient with 2 lesions in the bilateral middle cerebral arteries. In another patient with 2 lesions, stent success was obtained in 1 lesion in the vertebral artery, but not in another lesion in the middle cerebral artery. The failure was due to the stent system not being able to negotiate severe tortuosity proximal to the lesion. The same failure occurred in the other 3 patients with single lesions. Thus, the overall stent success rate for lesions was 91.7% (44/48). In the 44 lesions that obtained stent success, 5 had 2 stents placed and 39 had a single stent. Univariate analyses showed that severe tortuosity access and >10-mm-long lesion might be associated with stent failure (Table). Analysis stratified by lesion length revealed a significant relationship between severe tortuosity and stent failure (Cochran-Mantel-Haenszel, χ2 = 5.831; P = .016). Analysis stratified by access did not demonstrate a significant relationship between long lesions and stent failure.
Within 30 days, 4 (8.7%) of 46 patients had periprocedural complications, and no deaths occurred. The complications included 3 minor perforator strokes (6.5%, 3/46) at day 1 (resulting from the compromise of perforating arteries by stent placement) and an acute in-stent thrombosis soon after stent placement (2.2%, 1/46). By intra-arterial thrombolysis, the patient with acute thrombosis obtained early complete recanalization and returned entirely to preoperative status within 27 minutes from the onset, without bleeding complication or a new infarct on brain CT. The event was classified as ECER. Univariate analysis did not show any 1 of the 5 angiographic characteristics of stenoses associated with 30-day stroke and death (Table).
All patients were available for follow-up, which varied from 1 to 30.7 months (median, 23.9 months). In 46 patients, all had 30-day follow-up, 45 had 6-month follow-up, 44 had 12- and 18-month follow-up, and 24 had follow-up of ≥24 months. Total aggregate follow-up yielded 92.1 patient years. During the follow-up period, 1 patient (2.2%, 1/46) developed minor ischemic stroke in the target-lesion artery territory at 6.7 months, which was related to angiographically verified restenosis. In addition, 2 patients (4.8%, 2/42) had minor ischemic strokes in the nontreated artery territory occurring at 1.5 and 18.7 months, respectively. We noted 1 death at 10 months, resulting from pneumonia.
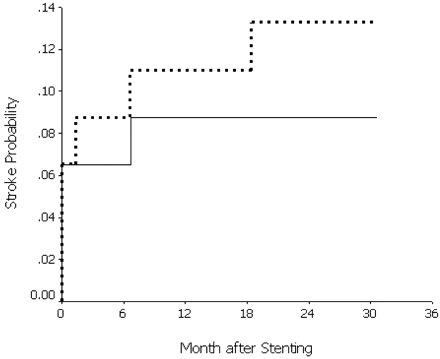
Stroke rates per 100 patient years were 4.3 for ischemic strokes in the target-lesion artery territory, including any stroke and death within 30 days, and 6.5 for ischemic strokes in any vascular territory, including any stroke and death within 30 days. Cumulative probability of ischemic strokes in the target-lesion artery territory, including any stroke and death within 30 days, was 8.8% at years 1 and 2. Cumulative probability of ischemic strokes in any vascular territory, including any stroke and death within 30 days, was 11.0% at 1 year and 13.3% at 2 years, shown in Fig 2.
Product-limit estimate of the cumulative probability of ischemic stroke after intracranial stent placement. Solid line shows ischemic stroke probability in the target-lesion artery territory, including any stroke and death within 30 days. Dashed line shows ischemic stroke probability in any vascular territory, including any stroke and death within 30 days.
Angiographic follow-up was performed in 25 patients (25 stented vessels). At the median time of 7.4 months (range, 3–17.1 months), 7 restenoses (28%, 7/25) were detected, including 6 asymptomatic ones and 1 symptomatic one.
Discussion
The ASSIST study shows that angioplasty with the Apollo stent for selected patients with SAIS is technically feasible. The SSYLVIA study (using Neurolink, a balloon-expandable intracranial stent) and another study (using Wingspan, a self-expandable intracranial stent) also demonstrated high stent success rates.15,16 At present, neither the Neurolink nor Wingspan stent is available in our country. It is difficult for us to compare empirically characteristics of the Apollo stent with those 2 stents. Nevertheless, this study determines severe tortuosity to be an independent predictor of stent failure with the balloon-expandable Apollo stent system.
Clinical outcome of the current study seems to compare favorably with the results of antithrombotic therapy. The conclusion from the WASID trial was that aspirin should be used in preference to warfarin for patients with SAIS, because warfarin is associated with significantly higher rates of adverse events and provides no benefit over aspirin. However, the trial found a 12% 1-year and a 15% 2-year probability for recurrent stroke in the territory of the stenotic artery in patients treated with aspirin, and an 8.3 per 100-patient-years' ischemic stroke rate in the territory of the stenotic artery (42/504 patient years of follow-up).5 The trial also demonstrated a significantly higher risk of subsequent stroke in the territory of the stenotic artery in patients with ≥70% stenosis than in patients with <70% stenosis.24 A total of 25 lesions (52.1%) in the current study had ≥70% stenosis, whereas only 37.7% of the lesions in the aspirin group of the WASID trial had >70% stenosis.5 Although there was a higher proportion of ≥70% stenosis in our patients, cumulative probability of ischemic stroke in the target-lesion artery territory, including any stroke and death within 30 days, was 8.7% at years 1 and 2, and the stroke rate was 4.3 per 100 patient years.
The ASSIST study also showed that perioperative strokes account for most of primary end point events. Therefore, reducing the risk of the procedure-related strokes should be the most crucial problem, which is related to whether patients benefit from stent placement or not. Currently, perforator stroke remains a challenge of stent placement for intracranial atherosclerosis. Compromise of perforating arteries after intracranial angioplasty or stent placement may be associated with perforator stroke.25,26 Our previous study revealed that patients with preoperative perforator infarct adjacent to the stenotic segment have a significantly higher perforator stroke frequency (8.2% versus 0.8%, P = .031) after elective stenting of intracranial stenosis.27 Identifying such a high-risk patient in advance might be mandatory to reduce stroke risk. In-stent acute thrombosis is still a common complication related to local intimal injury after angioplasty or stent placement, despite pretreatment with aspirin and clopidogrel. Whether timely revascularization of an acute occlusion will bring a completely different outcome is uncertain. The excellent outcome of the patient with this complication in this series shows the importance of early complete recanalization of an acute thrombosis.
Similar to 32.4% in the SSYLVIA study,15 a higher restenosis frequency of 28% was observed in this study. Fortunately, most restenoses were asymptomatic. This condition may partially be explained by restenosis caused by neointimal proliferation with little potential for thromboembolism or development of a collateral blood supply.24
There are certain limitations in this study. First, being from a single center, our results may not be generalizable for reasons of interventional neuroradiologists' experience and multidisciplinary care. Second, the restenosis rate may be over- or underestimated because only half of the patients had angiographic follow-up studies. Last, it is difficult to show a definitely beneficial effect of stent placement in this pilot study because we did not compare stent placement with medical therapy.
Conclusion
Angioplasty with the Apollo stent for symptomatic atherosclerotic intracranial stenosis is feasible, and severe tortuosity is an independent predictor of stent failure. Our clinical outcome seems to compare favorably with the results of aspirin therapy, but the restenotic rate is high. Randomized trials comparing stent placement with medical therapy are needed.
Footnotes
This work was supported by the Ministry of Health of The People's Republic of China (2004BA714B-7).
References
- Received June 28, 2006.
- Accepted after revision August 27, 2006.
- Copyright © American Society of Neuroradiology