Abstract
BACKGROUND AND PURPOSE: There are, to our knowledge, no histologic data correlating aneurysm volume with histologic healing following coil embolization of aneurysms. We report a retrospective study comparing histologic outcome with aneurysm volume in elastase-induced aneurysms in rabbits.
MATERIALS AND METHODS: Aneurysm volume and histologic healing after coil embolization were retrospectively analyzed in 37 elastase-induced aneurysms in rabbits. Aneurysm dimensions (including neck, width, height, and volume) were measured and calculated. Packing density (PD) was calculated. Angiographic results were evaluated as recurrence, stable, and progressive occlusion. An ordinal grading system was used to evaluate the histologic healing after embolization. Correlations among aneurysm volume, PD, and histologic healing were analyzed by conducting linear regression analysis.
RESULTS: For all the aneurysms in this study, mean aneurysm volume was 80.8 ± 48.6 mm3 (from 22 to 192 mm3), mean PD was 30.4 ± 8.3% (from 17% to 49%), and mean histologic score was 6.1 ± 2.0 (from 0.5 to 9.5), respectively. Correlations between aneurysm volume and PD, aneurysm volume and histologic healing, and aneurysm packing and histologic healing were all significant (P < .01).
CONCLUSION: In this study, aneurysms with smaller volumes and higher PD were associated with the most complete histologic healing. The incomplete healing seen in the larger aneurysms is consistent with the higher incidence of recurrences after endovascular treatment that is seen in large human aneurysms.
Endovascular coil embolization has been widely used as an effective method for the treatment of intracranial aneurysms.1–5 However, incomplete embolization, aneurysm recanalization, and coil compaction remain commonplace.6,7 The angiographic outcome after coil embolization in small aneurysms is superior to that of large and giant aneurysms.8–10 However, histologic studies correlating aneurysm volume and healing have not previously been reported. In this study, we correlated the histopathologic features of embolized aneurysms with aneurysm volume in an elastase-induced aneurysm model in rabbits.
Materials and Methods
Aneurysm Creation
Thirty-seven embolized aneurysms in New Zealand white rabbits (body weight, 3–4 kg) were retrospectively analyzed in this study. The Institutional Animal Care and Use Committee at this institution approved all procedures. Detailed procedures for aneurysm creation have been described elsewhere.11 Briefly, the rabbits were anesthetized with an intramuscular injection of ketamine, xylazine, and acepromazine (75, 5, and 1 mg/kg, respectively). A 5F sheath (Cordis Endovascular, Miami Lakes, Fla) was advanced retrograde in the right common carotid artery (RCCA) to a point approximately 3 cm cephalad to the RCCA origin. A 3F Fogarty balloon (Baxter Healthcare, Irvine, Calif) was advanced to the origin of the RCCA at its junction with the subclavian artery. Porcine elastase was incubated in the dead space of the RCCA, above the inflated balloon, for 20 minutes. The incision was sutured closed, and the animal was permitted to recover. Aneurysms were allowed to mature for at least 3 weeks after creation to ensure aneurysm cavity stability.12
Embolization Procedure
The details of the embolization procedure have been described elsewhere.13 Briefly, anesthesia was induced and maintained as it was during aneurysm creation. Surgical exposure of the right common femoral artery (CFA) was performed, and a 5F vascular sheath was placed. A 5F catheter (Envoy; Cordis Endovascular) was advanced into the brachiocephalic artery. Using a coaxial technique, we advanced an Excel microcatheter (Boston Scientific, Natick, Mass) into the aneurysm. The size of the aneurysm was assessed by direct comparison with radiopaque sizing devices. All the aneurysms were embolized as densely as possible by using bare platinum coils.
Before and after coil embolization, intra-arterial digital subtraction angiography (IADSA) of the parent artery and aneurysm was performed. After angiography, the catheters and vascular sheath were removed, and the proximal aspect of the femoral artery was ligated with a 4–0 silk suture. The incision was closed with a 4–0 Vicryl running suture (Ethicon, Somerville, NJ), and the animal was permitted to recover.
Packing Density Calculation
Packing density (PD, percentage) was calculated by using the method previously described.14 Briefly, the aneurysm volume was calculated by using the following formula: 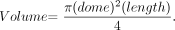 The value of the aneurysm dome in this formula is equal to that of aneurysm width. The algebraic equation used to calculate the volume of the coil was coil volume = 3.14 × (diameter of coil/2)2 × length of coil. Coil PD was calculated as PD = (volume of coil / volume of aneurysm) × 100%.
The value of the aneurysm dome in this formula is equal to that of aneurysm width. The algebraic equation used to calculate the volume of the coil was coil volume = 3.14 × (diameter of coil/2)2 × length of coil. Coil PD was calculated as PD = (volume of coil / volume of aneurysm) × 100%.
Angiographic Evaluation
Two types of IADSA were performed at the time of sacrifice for follow-up. In 33 aneurysms, surgical access of the left CFA was achieved, in a fashion similar to that used for access of the right CFA described previously. A 5F catheter was placed in the brachiocephalic artery, and IADSA was performed. In the remaining 4 aneurysms, IADSA through the left ear artery was performed. Briefly, a 24-gauge angiocatheter was placed in the central artery of the left ear. IADSA at 2 frames per second was performed after injection of 7-mL iodinated contrast material (iohexol; Omnipaque 300, Nycomed, Princeton, NJ) through the angiocatheter for 2 seconds, and the injection rate was approximately 3 mL/s.
Postembolization and follow-up angiograms for each aneurysm were compared to determine the durability after embolization. Using the evaluation criteria published previously,14 1 blinded experienced observer evaluated 3 categories: aneurysm recurrence, stable, and progressive occlusion. “Recurrence” was defined as any interval increase in contrast opacification of the aneurysm cavity or neck between the immediate postembolization and the follow-up angiogram. “Progressive occlusion” was defined as any decrease in contrast opacification of the aneurysm cavity or neck between those 2 angiograms.
Histologic Preparation and Analysis
Aneurysms were harvested 1 month (n = 11), 4 months (n = 10), 6 months (n = 12), and 1 year (n = 4) after embolization. Details of the procedure have been described elsewhere.15 Briefly, at the time of sacrifice, the subjects were deeply anesthetized as described previously. Subjects were sacrificed by using a lethal injection of pentobarbital. The chest cavity was opened. The aneurysms were removed and fixed in 10% neutral buffered formalin immediately. The specimens were embedded in paraffin, and subsequently sectioned at 1000-μm intervals through the portion bearing metallic coils in a coronal orientation. The coil fragments were removed from these thick sections by using a dissecting microscope. After all coils were removed, the sections were re-embedded in paraffin and sectioned at 5-μm intervals. Hematoxylin-eosin (H&E) stain was used for conventional histopathologic evaluation. Sections were viewed by 1 blinded experienced reviewer. An ordinal grading system16 was used to evaluate findings on H&E-stained slides. Neck healing was based both on gross and microscopic inspection. The scores of the gross (0–3) and microscopic inspections (0–4) were averaged to yield a single neck score. If different areas of the aneurysm neck showed different levels of healing, intermediate scores (0.5) were used. Microcompaction assessment (0–3) was based on the shape of the coil mass across the neck, from concave to convex. Healing characteristics in the dome (0–5) were categorized on the basis of the attenuation of cellular infiltration and area of organized tissue. All these scores were added together to obtain a total score.
Statistical Analysis
Correlations between aneurysm volume and PD, aneurysm volume and histologic healing, and aneurysm packing and histologic healing were analyzed by using bivariate fit analysis (JMP; SAS, Cary, NC), which uses a simple linear regression. In addition, a stepwise regression was performed to tease out the factors examined in this study that best predicted histologic healing.
Results
Mean values for aneurysm neck size, width, and height were 3.0 ± 0.9 mm (range, 1.3–4.9 mm), 3.4 ± 0.8 mm (range, 2.2–5.1 mm), and 8.0 ± 1.8 mm (range, 4.7–11.2 mm), respectively. The mean aneurysm volume was 80.8 ± 48.6 mm3 (range, 22–192 mm3), the mean PD was 30.4 ± 8.3% (range, 17%–49%), and the mean histologic score was 6.1 ± 2.0 (range, 0.5–9.5).
The Impact of Aneurysm Volume on PD
The linear regression showed a significant (P < .001) negative correlation between aneurysm volume and PD (Fig 1). It indicated that as the aneurysm volume got larger, the PD decreased.
The graph illustrates a linear regression showing a significant (P < .001) negative correlation between aneurysm volume and PD. The graph also contains the regression line (straight line) and the 95% confidence intervals (bowed lines).
The Impact of Aneurysm Volume on Histologic Healing
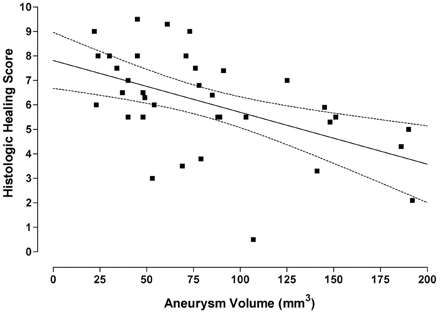
A significant (P < .01) inverse relationship between the histologic healing index and the aneurysm volume was shown in Fig 2. Complete thrombus organization within the aneurysm cavity was found more frequently in aneurysms with smaller volumes than in aneurysms with larger volumes. In addition, the aneurysms with the lower volumes tended to have endothelium lining the neointima across the neck, whereas the larger ones did not (Figs 3 and 4).
This graph illustrates a linear regression showing a significant (P < .01) inverse relationship between the histologic healing index and the aneurysm volume. The graph also contains the regression line (straight line) and the 95% confidence intervals (bowed lines).
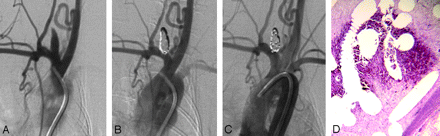
Healing in a larger aneurysm. A, DSA image before embolization shows the aneurysm cavity. B, DSA image immediately postembolization shows complete occlusion. C, DSA image before sacrifice shows that the aneurysm remains occluded. D, Staining shows that part of the aneurysm cavity is filled with unorganized thrombus (H&E, original magnification ×40).
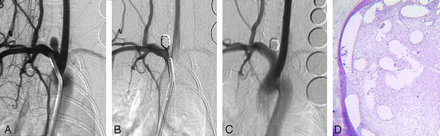
Healing in a smaller aneurysm. A, DSA image before embolization shows the aneurysm cavity. B, DSA image immediately postimplantation shows that the aneurysm is totally occluded. C, DSA image before sacrifice shows that the aneurysm remains completely occluded. D, Staining shows that the aneurysm dome is filled with attenuated connective tissue (H&E, original magnification ×40).
The Impact of PD on Histologic Healing
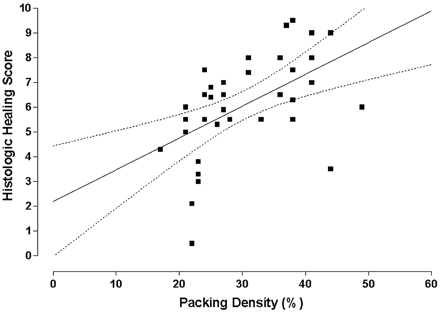
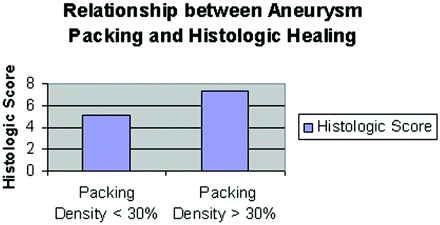
A linear regression indicated a significant (P < .01) positive correlation between histologic score and PD (Fig 5), indicating that higher PDs were related to higher histologic scores. The data were then broken out into PDs of <30% and ≥30%. The mean histologic score of the 20 aneurysms with PD < 30% was 5.1, which was lower than the mean score, which was 6.1 ± 2.0. Three aneurysms with a PD < 30% showed recurrence. The mean histologic score of the 17 aneurysms with PD > 30% was 7.3 (Fig 6). One aneurysm with a high PD (44%) showed progressive occlusion from digital subtraction angiography (DSA). All the other aneurysms remained stable after embolization.
This graph illustrates a linear regression showing a significant (P < .01) positive correlation between PD and histologic healing. The graph also contains the regression line (straight line) and the 95% confidence intervals (bowed lines).
A bar graph shows the relationship between PD versus histologic healing at >30% and <30%.
Discussion
Although endovascular treatment of intracranial aneurysms has been widely used because of its efficacy and safety,1–3,5 long-term follow-up studies have shown that aneurysm recanalization and/or regrowth remains a substantial problem, especially for large and giant aneurysms.2,3,6 Difference in histologic healing between small and large aneurysms may enhance our understanding of aneurysm healing, but no such histologic data have yet been published.
Our study indicated aneurysm volume was negatively correlated with PD. PD in large aneurysms was relatively lower when compared with that of smaller aneurysms. This is consistent with clinical results that indicate that loose packing can be found more frequently in larger and giant aneurysms.
Our data using an animal model showed that histologic healing was negatively correlated with aneurysm size (ie, smaller aneurysms healed more completely than larger aneurysms). Both the occurrence rate of complete thrombus organization within the aneurysm cavity and neointima across the aneurysm neck in smaller aneurysms were higher than those in larger aneurysms. The relatively poorer healing of larger aneurysms can be used to explain the clinical results of embolized large and giant aneurysms.
PD has been widely accepted as an important determinant of follow-up after coil embolization.8–10,17,18 The current study showed better healing in smaller aneurysms with higher PD. Conversely, larger aneurysms with lower PD indicated poorer healing. Thus, this study suggests that PD is correlated with aneurysm healing after embolization and poor histologic healing was linked to aneurysm recurrence. The same poor healing applies to large aneurysms in humans after coil embolization.19,20 However, because PD is inversely proportional to aneurysm volume, we cannot separate out the relative effects of PD and aneurysm volume on healing—that is, improved healing in smaller aneurysms may result from either higher PD or from some other feature of small aneurysms. These features may include relatively smaller distances for cells, both from the aneurysm wall and adjacent parent artery, to traverse into smaller aneurysms than into in larger aneurysms. However, it is interesting to note that in the relatively smaller aneurysms created in the animal model of this study, the same relationship of aneurysm size to histologic healing holds true.
The following limitations occurred in this study: First, the numbers of aneurysms were not large. Second, different time points for duration of implantation were used, ranging from 1 month to 1 year. However, a previous study has shown relatively little interval change in healing beyond 1 month using this model.16
Conclusion
Aneurysm volume is correlated with PD and histologic healing after coil embolization. Aneurysms with smaller volume and higher PD have better histologic outcomes. The poorer healing seen in the larger aneurysms is consistent with the higher incidence of recurrences after coil embolization in large human aneurysms.
Footnotes
This study was supported by the National Institutes of Health grant NS42646.
Paper previously presented at: 44th Annual Meeting of the American Society of Neuroradiology, April 29–May 5; 2006, San Diego, Calif.
References
- Received March 31, 2007.
- Accepted after revision May 27, 2007.
- Copyright © American Society of Neuroradiology