Abstract
SUMMARY: We assessed the influence of inclusion (method 1) and exclusion (method 2) of intratumoral vessels when determining maximum relative cerebral blood volume (rCBVmax) in 3 types of low-grade gliomas (LGGs): astrocytomas, oligoastrocytomas, and oligodendrogliomas. Method 1 yielded significantly higher mean rCBVmax than method 2. However, only method 2 demonstrated a significant (P = .026) association between rCBVmax and membership of a differently ranked histologic category. Exclusion of intratumoral vessels appears, therefore, preferable when determining rCBVmax in LGGs.
Dynamic susceptibility contrast-enhanced (DSC) MR perfusion imaging has become an important technique for studying brain tumors. Intratumoral relative cerebral blood volume (CBV [rCBV]) correlates with histologic and angiographic measures of tumor vascularity,1 helps predict the histologic grade of gliomas,2 and correlates with outcome in low-grade gliomas (LGGs).3 High-grade gliomas usually have a higher rCBV than low-grade tumors, but rCBVs may also be elevated in low-grade oligodendrogliomas (ODs),4 which confounds tumor grading.5
Techniques of data acquisition, postprocessing, and analysis influence rCBV measurements. Gradient-echo echo-planar imaging (GE-EPI) pulse sequences are more sensitive to larger vessels than spin-echo echo-planar imaging (SE-EPI) methods and provide better differentiation between histopathologic tumor grades.6
Wetzel et al7 found that interobserver and intraobserver reproducibility for intratumoral rCBV were best when the highest CBV from several regions of interest (ROIs) was chosen and highlighted the importance of excluding large vessels. Inclusion or exclusion of intratumoral vessels is often not explicitly stated in glioma perfusion studies, though some investigators focused on excluding peritumoral vessels.6 The significance of intratumoral vessels for rCBV measurements has, to our knowledge, not been formally examined.
Here we studied the influence of intratumoral vessels on rCBV characterization in 3 histologic categories of low-grade glial tumors: astrocytomas (ACs), ODs, and oligoastrocytomas (OAs).
Technique and Results
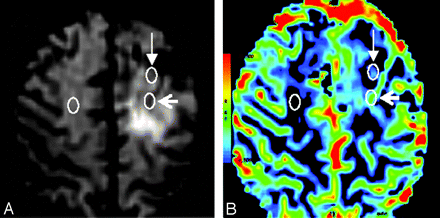
Thirty-four patients with LGGs, composed of 21 ACs, 8 ODs, and 5 OAs, had DSC GE-EPI (TR = 1200 ms; TE = 40 ms; flip angle = 20°; FOV = 26 cm; matrix = 96 × 128; section thickness = 5 mm) at 1.5T (GE Signa Horizon Echospeed LX9.1; GE Healthcare, Waukesha, Wis) with a bolus of 0.1 mmol/kg of body weight of gadoterate meglumine at 5 mL/s. Color maps of rCBV were generated with FuncTool 1.9 (GE Healthcare) and analyzed by 2 neuroradiologists (H.R.J. and G.B.C., with 15 and 7 years of experience, respectively), reaching a consensus for placement of ROIs. At least 6 intratumoral ROIs with a size of 9 pixels were placed over areas showing mostly elevated CBV on color perfusion maps. Blood vessels within the tumor were identified on unprocessed perfusion images acquired between the time points of maximum arterial and venous signal intensity drop. Sections above and below intratumoral vessels were viewed to identify potentially confounding large-vessel partial volume effects. We used 2 different methods for selecting the ROI with the maximum intratumoral CBV: method 1 included and method 2 excluded ROIs situated over intratumoral blood vessels and associated partial volume effects. The maximum rCBV (rCBVmax) was then obtained by dividing the highest intratumoral CBV by the mean CBV obtained from a contralateral normal-appearing white matter ROI (Fig 1).
T2*-weighted image during maximum arterial signal intensity drop (A) and rCBV map (B) in low-grade OD, demonstrating the position of ROIs used to calculate rCBVmax. Method 1 (open arrowhead, posterior ROI in the left cerebral hemisphere, overlying an intratumoral vessel) and method 2 (closed arrowhead, anterior ROI in the left cerebral hemisphere, lying outside intratumoral vessels). The mean CBV from contralateral normal-appearing white matter (ROI in the right cerebral hemisphere) was used to normalize the data for each method. White circles have been superimposed on the original color-coded ROIs generated by FuncTool.
Mean rCBVmaxs obtained for each group with each method are shown in the Table. Method 1 yielded higher mean values and wider ranges than method 2 in all 3 of the histologic tumor types, particularly in OA and OD. For the patient group as a whole, there was a significant difference between mean rCBVmax obtained using each method (P < .001, Wilcoxon test). Ordinal regression was used to assess the relationship of rCBVmax and tumor histology, categorized in 3 groups (0: OA; 1: AC; 2: OD). Only method 2 showed a significant association between rCBVmax and the risk of being in a histologic category with a higher ordinate. Using method 2, the odds ratio of being in a higher category was 4.25 (95% confidence interval: 1.19-15.14 for each additional unit rCBV increment; P = .026). Method 1 did not demonstrate a significant association between rCBVmax and the risk of being in a higher category (P = .638).
CBVmax obtained using methods 1 and 2
Discussion
Several studies have demonstrated increased rCBV in high-grade gliomas compared with low-grade tumors.2,4–6,8–10 There is, however, considerable variation in the reported rCBV values for low- and high-grade tumors (On-line Table). In SE-EPI DSC imaging, transverse relaxation rates peak at a vessel diameter of 1-2 μm, whereas in GE-EPI DSC imaging they plateau at 3–4 μm and then remain independent of vessel size, explaining the lower rCBVs found with SE techniques.6 Differences may also be due to variations in the histologic types of LGG examined and inclusion or exclusion of intratumoral vessels in the analysis, often not specified.
We demonstrated that inclusion of large intratumoral vessels significantly increases rCBVmax values in all types of LGG. Their identification may be difficult on rCBV color maps alone and necessitates reviewing of unprocessed perfusion data. The diameter of intratumoral vessels clearly identifiable on GE-EPI images lies in the millimeter range (approximating the size of peripheral leptomeningeal vessels), whereas glioma neoangiogenetic vessels in animal models measure between 40 and 250 μm.6
In concordance with previous investigators, we found higher rCBVmax in tumors with oligodendral elements than in purely astrocytic tumors,5 explained by the “chicken wire” hypervascularity seen in the former. Cha et al4 chose intratumoral ROIs with an automated method targeting areas of maximum signal intensity decrease during the first pass of the gadolinium-based contrast bolus. This method probably incorporated intratumoral vessels and yielded mean rCBV for ODs of 3.68. Spampinato et al8 presented one of the few reports specifying exclusion of large intratumoral vessels for ROI analysis. Their mean rCBV measurement for a mixed group of low-grade OA and OD (1.61) lies between our group mean rCBV measurements of OA (1.53) and OD (2.21) using method 2. Our findings highlight the importance of using a consistent ROI placement technique, particularly if rCBV data are to be pooled in multicenter studies. The relationship between rCBVmax on MR perfusion imaging and histologic classification as OA versus OD has not been reported previously. We were able to demonstrate significant association between maximum intratumoral rCBV values and histopathologic classification as AC, OD, and AO, but only when using method 2. As a preferred technique we, therefore, recommend exclusion of intratumoral vessels when determining maximum intratumoral rCBV from GE-EPI DSC-derived data. Although beyond the scope of this work, future research will address the influence of intratumoral vessels on rCBV measurements in high-grade gliomas, which are naturally subject to greater variability.
Footnotes
indicates supplemental on-line table.
References
- Received June 20, 2007.
- Accepted after revision September 10, 2007.
- Copyright © American Society of Neuroradiology