Abstract
BACKGROUND AND PURPOSE: Cerebral and cervical arterial abnormalities are the most common non-cutaneous anomaly in PHACE syndrome, but the location and type of arterial lesions that occur have not been systematically assessed in a large cohort. Our aim was to characterize the phenotypic spectrum of arteriopathy, assess the frequency with which different arteries are involved, and evaluate spatial relationships between arteriopathy, brain structural lesions, and hemangiomas in PHACE syndrome.
MATERIALS AND METHODS: Intracranial MRA and/or CTA images from 70 children and accompanying brain MR images in 59 patients with arteriopathy and PHACE syndrome were reviewed to identify the type and location of arterial lesions and brain abnormalities. Five categories of arteriopathy were identified and used for classification: dysgenesis, narrowing, nonvisualization, primitive embryonic carotid-vertebrobasilar connections, and anomalous arterial course or origin. Univariate logistic regression analyses were performed to test for associations between arteriopathy location, hemangiomas, and brain abnormalities.
RESULTS: By study design, all patients had arterial abnormalities, and 57% had >1 form of arteriopathy. Dysgenesis was the most common abnormality (56%), followed by anomalous course and/or origin (47%), narrowing (39%), and nonvisualization (20%). Primitive embryonic carotid-vertebrobasilar connections were present in 20% of children. Hemangiomas were ipsilateral to arteriopathy in all but 1 case. The frontotemporal and/or mandibular facial segments were involved in 97% of cases, but no other specific associations between arteriopathy location and hemangioma sites were detected. All cases with posterior fossa anomalies had either ICA anomalies or persistent embryonic carotid-basilar connections.
CONCLUSIONS: The arteriopathy of PHACE syndrome commonly involves the ICA and its embryonic branches, ipsilateral to the cutaneous hemangioma, with dysgenesis and abnormal arterial course the most commonly noted abnormalities. Brain abnormalities are also typically ipsilateral.
Abbreviations
- ACA
- anterior cerebral artery
- AcomA
- anterior communicating artery
- AP
- anteroposterior
- BA
- basilar artery
- CTA
- CT angiography
- ICA
- internal cerebral artery
- MCA
- middle cerebral artery
- MRA
- MR angiography
- OMIM
- Online Mendelian Inheritance in Man
- PCA
- posterior cerebral artery
- PcomA
- posterior communicating artery
- PHA
- persistent hypoglossal artery
- PSA
- persistent stapedial artery
- PTA
- persistent trigeminal artery
- VA
- vertebral artery
The acronym PHACE (OMIM #606519) summarizes the features of an uncommon neurocutaneous syndrome in which infantile Hemangiomas of the head and neck are found in association with Posterior fossa malformations, Arterial anomalies, Cardiac defects, and/or abnormalities of the Eye.1 Cervical and intracranial arteriopathy has been reported as the most common extracutaneous abnormality in this disorder, occurring with an estimated prevalence of 84% in a national registry of patients.2 The range of arterial anomalies includes agenesis, luminal narrowing, dolichoectasia, persistence of primitive embryonic arteries, aneurysms,3–7 and, in a small number of subjects, progressive postnatal narrowing, Moyamoya-like collaterals, and thrombosis.6,8,9 We retrospectively reviewed abnormal angiographic cross-sectional imaging studies in 70 children meeting consensus criteria for PHACE syndrome to achieve the following objectives: 1) characterize the phenotypic spectrum of arteriopathy in the head and neck, 2) assess the frequency with which different arteries are involved, and 3) explore relationships between arteriopathy, segmental hemangiomas, and structural brain lesions in the context of embryologic hypotheses that have been proposed for the development of the disorder.
Materials and Methods
This study was performed under a research protocol approved by the Committee on Human Research at the University of California, San Francisco and by institutional review at participating sites. Criteria for inclusion were the following: 1) a clinical diagnosis of PHACE syndrome according to recently proposed diagnostic criteria,10 and 2) abnormal brain and/or neck arterial imaging findings according to consensus interpretation by 2 neuroradiologists.
Patients
The population considered for inclusion in this study consisted of a historic cohort of 91 individuals with large segmental hemangiomas of the head and neck (>5 cm in diameter) and clinically diagnosed PHACE syndrome. Subjects were evaluated in pediatric dermatology clinics at 4 participating academic institutions during a 10-year period from 1999 to 2008. Age and sex were recorded at each site, along with the results of a dermatologic examination that included an assessment of the hemangioma locations according to the segment map of Haggstrom et al.11 Although all study subjects underwent cross-sectional angiographic imaging to exclude arterial abnormalities, only 70 patients had arterial lesions in the brain, head, and/or neck that met inclusion criteria (see below). Twenty-two of these subjects were concurrently enrolled in a separate study assessing the risk of PHACE syndrome in high-risk infants with facial hemangiomas of >24 cm2.12 The clinical aspects of some cases included in this study have also been previously reported.9,13
Radiology Review
Imaging was performed according to various protocols at 17 different institutions and imaging facilities. Because many subjects initially underwent imaging locally before being referred to 1 of the 4 primary treating institutions, image quality varied depending on specific acquisition parameters, magnetic field strengths, and the presence of artifacts. All examinations included either time-of-flight MRA or bolus CTA images suitable for evaluation of the upper cervical and intracranial arterial system around the skull base and circle of Willis. Among the 91 imaging studies initially reviewed, 21 were excluded for the following reasons: common normal variations in the circle of Willis without other arterial anomalies (15 subjects), insufficient spatial coverage (2 subjects), or poor technical scan quality (4 subjects).
Detailed analysis of arteriopathy type and location was then performed on the remaining 70 patients by 2 subspecialty-certified neuroradiologists by using MRA (59 subjects), CTA (3 subjects), or both (8 subjects). All 59 patients with MRA also had conventional brain MR imaging that included at least T1-, T2-, and gadolinium-enhanced T1-weighted sequences. These images of the brain were assessed for the presence or absence of infarcts and/or other supra- and infratentorial structural abnormalities that have been previously associated with PHACE sydrome.2–4,7,10,13–15
Classification and Analysis of Arterial Abnormalities
On the basis of prior studies of arterial anomalies in PHACE sydrome,3–9 we used 5 categories to classify arteriopathy on cross-sectional imaging:
“Dysgenesis” was used to describe bizarre looping, elongation, ectasia, kinking, and focal or fusiform aneurysmal enlargement.
“Narrowing” was characterized as either focal or long-segment (≥1 cm). Arterial stenosis was not distinguished from developmental hypoplasia because time-of-flight MRA did not reliably allow this distinction.
“Nonvisualization” was defined as the absence of a normal artery. Similar to the case of narrowing, arterial agenesis, occlusion, or severe stenosis was considered as a single category because time-of-flight MRA did not consistently allow this distinction.
“Persistent embryonic carotid-vertebrobasilar arterial connections” included PTA, PSA, and PHA. No cases of persistent otic or proatlantal segmental arteries were observed.
“Abnormalities in arterial course and/or origin” were assigned when there were significant differences from conventional arterial branching, including persistent embryonic arteries other than carotid-vertebrobasilar connections.
Only abnormalities in the large and medium cerebral arteries (defined as the ICA, ACA, MCA, PCA, AcomA, PcomA, VA, or BA) were assessed because smaller more peripheral intracranial arteries were not reliably depicted on all studies. In addition to the type of arteriopathy, we also assessed the arteries involved and the laterality of arterial abnormalities with respect to the hemangioma (ipsilateral and/or contralateral). ICA arteriopathy was further categorized as segmental or pleurisegmental by using the following embryologic segments16: cervical, ascending and/or horizontal petrous, ascending and/or horizontal cavernous, or clinoid and/or terminal carotid.
The relative frequency of cases with abnormalities assigned to each category of arteriopathy and the different arteries involved was determined, and univariate logistic regression analyses were performed to assess the following: 1) whether unilateral arteriopathy was associated with the presence of an ipsilateral hemangioma, 2) whether bilateral arteriopathy was associated with bilateral hemangiomas, 3) whether the artery involved was associated with the segmental location of the observed hemangioma, and 4) whether the artery involved was associated with the presence of a brain structural abnormality. All statistical analyses were performed by using Stata, Version 9 (StataCorp, College Station, Texas).
Results
Patient Demographics
Similar to previous findings in PHACE syndrome,17 a significant difference in sex was noted, with 60 female and 10 male individuals showing imaging evidence for arteriopathy. Age at the time of imaging varied from 1 day to 11.4 years (mean, 7.2 months; interquartile range, 3.5 months to 1.7 years). Approximately 85% of subjects were younger than 2 years of age. The older ages reflected the fact that imaging performed at the time of initial diagnosis was not available for some patients.
Segmental Facial Hemangiomas
Segmental hemangiomas were more commonly unilateral (42/70 cases) than bilateral (28/70 cases). When unilateral, hemangiomas were present in nearly equal proportions on the right (22 cases) and the left (20 cases). In 48/70 cases, hemangiomas involved >1 facial segment. The S1 (frontotemporal) and/or S3 (mandibular) segments were involved in all but 2 cases; S1 segment hemangiomas, seen in 59/70 of subjects, were more common than S3 hemangiomas, which were present in 42/70 subjects. The S4 (frontonasal) segment was least frequently involved, present in 23/70 of cases. A single case involved the S2 (maxillary) segment alone. All except 1 S4 lesion were associated with an S1 lesion.
Arteriopathy
Among the different categories used for classification of arteriopathy, dysgenesis was the most common anomaly, present in 39/70 cases. Abnormalities in this category were also the most variable in appearance (Fig 1). Also included in this category were 10 aneurysms, all located within segments of arteries that also met the criteria for dysplasia. These were characterized morphologically as eccentric outpouchings in 2 cases, smooth fusiform enlargement in 4 cases, and irregular fusiform enlargement in 4 cases. Aneurysms involved the petrous or supraclinoid ICA (5 cases), the right precommunicating segment of the PCA (3 cases), the ACA (1 case), and the C2 segment of the vertebral artery (1 case).
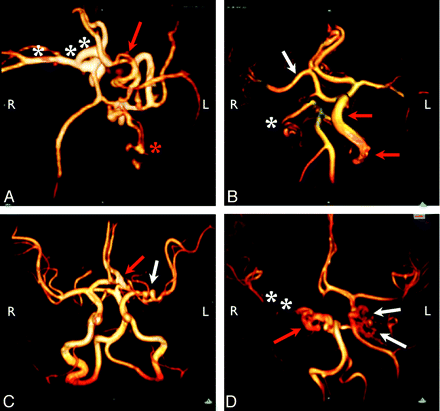
Representative arterial dysgenesis images are shown as 3D volume renderings from MRA source data, generated by using OsiriX (http://www.osirix-viewer.com/Downloads.html).32 A, Enlargement of the right ICA, MCA, and ACA (white asterisks). There is also an accessory left MCA (red arrow), and the left PCA is duplicated with an anomalous branching pattern (red asterisk). B, Proximal irregularity of a markedly enlarged left ICA (red arrows) and tortuosity of the right PCA (white asterisk). The origin of the right MCA (white arrow) is also anomalous, arising from the cavernous ICA. C, Looping course of the left M1 segment (white arrow) and enlarged left A1/A2 junction (red arrow). D, Marked dysgenesis of the cavernous and supraclinoid right ICA (red arrow) and contralateral dysgenesis of the cavernous and supraclinoid left ICA (white arrows). Note also that the right ICA is not visualized proximal to the cavernous sinus, the right PcomA supplies the right MCA, and the right MCA is markedly narrowed (white asterisks).
Abnormal arterial course or origin or both (Fig 2) were the second most common type of arteriopathy, observed in 33/70 cases. Some of these cases represented persistent embryonic arteries; others did not correspond to known embryonic arterial segments. For example, we encountered 7 cases with aberrant origin of the ophthalmic artery, which arose from the middle meningeal artery (3 cases), the ACA (2 cases), the cavernous segment of the ICA (1 case), or the BA (1 case), corresponding to persistence of primitive embryonic variants of this artery.18,19 In many cases, enlarged anomalous branches provided arterial supply to a large facial or scalp hemangioma.
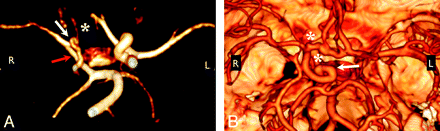
Anomalous course and/or origin. A, 3D rendering from MRA shows elongation of the right PcomA, an anomalous connection between the right P1 segment and the right MCA (red arrow). The right ICA and left PCA are also diffusely narrow (white arrow), and the right A1 segment is hypoplastic (white asterisk). B, 3D rendering from CTA shows the tortuous looped course of the supraclinoid right ICA (white arrow). Note also the presence of 2 ACAs, a separate artery of the corpus callosum, and aneurysms at the right carotid terminus and distal right A1 segment (white asterisks). Volume renderings were generated by using OsiriX.32
Embryonic carotid-basilar connections were present in 14/70 cases. PTA was most common (11 cases); PHA and PSA were observed in 1 and 2 cases, respectively.
Narrowing (Fig 3) was seen in 27/70 cases, involving a segment of ≥1 cm in length in all except 2 subjects. There were no cases of MCA or supraclinoid ICA narrowing associated with classic Moyamoya collaterals, though small collaterals were observed around segments of the ICA and MCA, together with focal dysgenesis and narrowing in 3 cases.
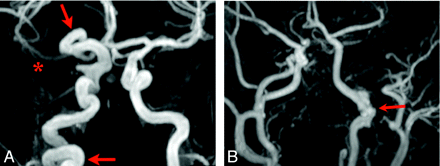
Arterial narrowing and nonvisualization. All images are shown as maximum-intensity-projection reconstructions from MRA source data. A, Submentovertex projection showing nonvisualization of the left ICA. B, AP projection shows long-segment narrowing of the entire visualized course of the right ICA (red arrow). C, AP projection shows nonvisualization of the left ICA from the distal cervical to the cavernous segments. The intradural left VA is also absent. D, AP projection shows absence of the entire left ICA and long-segment tapered narrowing and luminal irregularity of the distal cervical right ICA (red arrow).
Nonvisualization (Fig 3) was a feature in 14/70 cases. Although agenesis was not distinguished from occlusion in most cases with MRA, both were observed in the few patients who underwent CTA. A single case showed segmental agenesis of the supraclinoid ICA.5
Arteriopathy was more common in the anterior circulation than in the posterior circulation (Table 1). The ICA was the most commonly involved artery, followed by the MCA and ACA, the PCA, and, least commonly, the BA and VA. Abnormalities were most frequent in the cervical segment of the ICA, though other embryologic segments were involved in similar proportions. A majority of cases with ICA abnormalities (60%) showed pleurisegmental lesions (Fig 4). Univariate logistic regression analysis revealed no associations between the artery involved and the segmental location of the hemangioma.
Location of arterial abnormalities
Examples of segmental and pleurisegmental ICA dysgenesis. Lesions of the ICA, especially dysgenesis, narrowing, and nonvisualization, often involve the entire length of the artery (red arrows, A) or relatively long portions of the artery corresponding to developmental segments (red arrow, B). The right proximal MCA shows diminished flow-related enhancement as well (red asterisk, A). There is segmental dysgenesis of the petrous left ICA with irregular enlargement of the artery (B). Note also a markedly enlarged left ECA, which supplies a large hemangioma, and an absent right A1 segment.
Arteriopathy was also more commonly unilateral (54/70 cases) than bilateral (16/70 cases) and, when unilateral, occurred with similar frequency on the right (25/70 cases) and on the left (29/70 cases). Among the 54 cases of unilateral arteriopathy, 53 (98%) had ipsilateral hemangiomas. Bilateral arteriopathy was not associated with bilateral hemangiomas.
Infarcts
Three patients had remote infarcts on imaging. One had evidence of right MCA in utero ischemic injury, right frontal polymicrogyria, a right globe coloboma, and multiple arterial anomalies, including an absent left ICA, a large AcomA, and a persistent right hypoglossal artery. One had evidence of a perinatal stroke and hypoplasia of the left ACA and narrowing of the left MCA; an acute left MCA infarction had been previously diagnosed in this patient. The third had a remote left MCA and lateral lenticulostriate territory infarction on an MR imaging performed at 3.6 years of age; MRA in this patient revealed a thin left M1 segment of the MCA with corkscrew elongation.
Brain Structural Abnormalities
In the 59/70 patients with conventional MR imaging available for review, structural abnormalities were present in 41% (24/59). Both supra- and infratentorial lesions were observed (Table 2). All unilateral lesions were associated with both arteriopathy and hemangioma ipsilateral to the side of the brain abnormality on MR imaging.
Structural brain abnormalities
Posterior fossa abnormalities were the most common structural anomaly. Only 1 subject exhibited a classic Dandy-Walker malformation. In all other cases, unilateral cerebellar hemispheric hypoplasia was present with or without involvement of the vermis. The overlying bony posterior fossa did not appear abnormal in any cases. All except 1 unilateral posterior fossa lesion had either an abnormality of the ipsilateral ICA or a persistent embryonic carotid-basilar connection.
Supratentorial structural abnormalities included callosal dysgenesis and malformations of cortical development. The latter were all observed ipsilateral to absent or dysplastic segments of the ICA. Perhaps most characteristic of PHACE syndrome, homogeneously enhancing extra-axial lesions isointense to cutaneous hemangiomas on all sequences and presumed to represent intracranial hemangiomas14 were seen in this group of patients, though in only 4 subjects. These were located in the cavernous sinus or internal auditory canal ipsilateral to the observed side of arteriopathy.
Discussion
This work confirms a strong association between the laterality of head and neck arteriopathy, cutaneous hemangiomas, and brain structural abnormalities in patients with PHACE syndrome and emphasizes the significant proportion of cases in which the ICA or its early embryologic branches or both are involved in the disorder. While the findings in large part concur with current ideas regarding the etiology of PHACE syndrome, they also raise the novel possibility that the variable phenotype of the disorder could, in part, be accounted for by alterations in blood flow caused by the development of arteriopathy during embryogenesis.
We found that the 2 most common forms of arteriopathy were dysgenesis and anomalous arterial origin and/or course. Estimates of frequencies for different types of arteriopathy in this work differ from those given in prior studies6,7 in 3 important respects: First, arterial abnormalities were classified on the basis of primary review of cross-sectional imaging, rather than written descriptions extracted from medical records or published case reports. Second, the use of consensus criteria for the diagnosis of PHACE syndrome led to a more uniform group of patients for analysis. Finally, whereas prior estimates have been based on groups of patients both with and without cerebrovascular anomalies, our study was limited to only subjects with arteriopathy.
The frequent coexistence of different forms of arteriopathy suggests that the categories used for classification in this and other reports represent different pathologic end points of the same underlying process. The most likely candidate is a disruption in the normal histologic architecture of the arterial wall, which, as discussed by Bhattacharya et al,4 could result in reductions or increases in cross-sectional diameter, elongation, corkscrewing, and/or tortuosity. Corkscrewing and other forms of dysgenesis are exceedingly rare in children and are most often associated with disorders that have decreased elasticity of tissues such as Menkes disease.20 Long-segment concentric narrowing of the ICA is similarly unusual because most secondary childhood stenosing arteriopathies—eg, Moyamoya disease, sickle cell vasculopathy, focal arteriopathy of childhood, and dissection—tend to cause more focal narrowing. To the authors' knowledge, there have been no tissue biopsies of the abnormal cervical or intracranial arteries in patients with PHACE syndrome, though the reported microstructural abnormalities in the arterial intima and media of the diseased aorta in 2 children undergoing aortic coarctation repair21 support the idea of dysgenetic vessels. Anomalous arterial origin and course, which, in some cases, corresponded to persistent embryonic arteries, and primitive embryonic carotid-vertebrobasilar connections suggest a different process, in which the normal pattern of vasculogenesis is altered during development of the cervical and intracranial arterial system.
As elegantly summarized by Krings et al,22 current hypotheses regarding the developmental errors that link the abnormalities in PHACE syndrome have focused on the role of the neural crest, the adjacent cephalic mesoderm, and even the neural plate in cases of brain structural lesions. Chimera studies in the avian embryo provide evidence that the muscular and connective tissue wall of the ICA and its branches are derived from cephalic neural crest cells, in contrast to the arterial wall of the basilar and vertebral arteries, which derive from the cephalic mesoderm.23 The disproportionate frequency with which the anterior circulation is involved compared with the posterior circulation follows the embryologic division between neural crest and mesodermal-derived arteries. However, for reasons outlined by other authors, metameric disruption in the neural crest and adjacent cephalic mesoderm does not fully account for the spectrum of abnormalities in PHACE syndrome.4,19,22
The primitive ICA is vital to the normal development of the brain and surrounding structures because it is the embryologic precursor to the MCA, the ACA, the PcomA arteries, the PCAs, the superior cerebellar arteries, and the upper BA and it is the primary source of blood to the developing vertebrobasilar system. According to Padget,24 this artery is first seen as the cranial extension of the dorsal aorta at the 3-mm embryonic stage (22–23 days) and gives rise to its primary branches by the 16-to 18-mm stage (48–51 days). During this roughly 4-week period, the primitive ICA also supplies the posterior circulation through a series of transient intersegmental arteries that each normally develop and regress for 7–10 days until the vertebrobasilar system is fully developed. Blood supply to the cerebellum in particular is critically dependent on the ICA during this phase of embryogenesis.
While different brain structural anomalies observed in this study and previously associated with PHACE syndrome may individually be caused by a variety of factors, both the common posterior fossa abnormalities and the less frequent supratentorial abnormalities share in utero ischemia as a common potential cause. Before fusing and becoming the BA between 29 and 33 days' gestation, the paired dorsal longitudinal arteries are supplied mainly by the primitive ICA by means of the trigeminal arteries. It is not until nearly 6 weeks' gestation that the vertebral arteries become the major contributor to hindbrain blood supply. Compromised vascular blood flow within 1 longitudinal artery before this point could impair formation of the ipsilateral cerebellar hemisphere and vermis. Abrupt disruption of an otherwise normally developing cerebellum is suggested by the normal bony posterior fossa,4 consistent with an ischemic etiology for cerebellar anomalies. Similarly, transient or permanent vascular insult and in utero ischemia are well-established causes for polymicrogyria,25 callosal dysgenesis,26 heterotopia,27 cerebellar hypoplasia,28 and other malformations of cortical development.
Although almost all cases with intracranial arteriopathy have S1 or S3 segment hemangiomas, the lack of a significant association between the arteries involved and the segmental location of the facial hemangioma militates against a common metameric origin for the simultaneous development of both abnormalities. Recent work provides evidence that the endothelial progenitor cells that give rise to hemangiomas may ultimately derive from the neural crest,29 though the cellular and molecular events that would induce the neural crest to differentiate along these lines have not yet been elaborated. At birth, most hemangiomas represent “precursor lesions” that subsequently proliferate. Alterations in blood flow or hypoxia may serve as the trigger that leads to the development of latent hemangioma precursors. Hemangiomas at all stages of evolution express glucose transporter 1,30 an intrinsic cellular marker of tissue hypoxia, and the combination of hypoxia and estrogen demonstrates a synergistic effect on hemangioma endothelial cell proliferation.31
There are 2 important limitations of this study. First, because most patients underwent only MRA to study their arteries, it was not possible to distinguish between agenesis and occlusion or between stenosis and hypoplasia, which represent fundamentally different processes. This limitation hinders our ability to divide these anomalies from an embryologic standpoint. Second, the average age of children included in this work was relatively old with respect the diagnosis of PHACE syndrome. To the extent that arteriopathy may change with time, this ascertainment bias could lead to inaccuracies in the estimated frequencies of different types of arteriopathy.
Conclusions
Pleurisegmental dysgenesis, especially involving the ICA, and anomalous course and origin of the cervical and intracranial arteries, often representing persistence of embryonic arteries, are the 2 most frequent arterial anomalies observed on cross-sectional angiographic imaging in patients with PHACE syndrome. The phenotypic variability of brain structural lesions and hemangiomas could conceivably be explained by alterations in blood flow and, in some cases, hypoxia, as a result of arteriopathy during embryogenesis.
Footnotes
-
I.J.F. and A.J.B. contributed equally.
-
Indicates Fellows’ Journal Club selection
-

-
This work was supported by the American Society of Pediatric Neuroradiology Award in Pediatric Neuroradiology Research. Dawn Siegel is partially funded through the Oregon Clinical Translational Research Institute UL1 RR024140 01.
-
Paper previously presented in part at: 47th Annual Meeting of the American Society of Neuroradiology, May 16–21, 2009; Vancouver, British Columbia, Canada; and 18th International Workshop on Vascular Anomalies, April 21–24, 2010; Brussels, Belgium.
References
- Received February 11, 2010.
- Accepted after revision May 12, 2010.
- Copyright © American Society of Neuroradiology