Abstract
BACKGROUND AND PURPOSE: Parathyroid gland weight is a clinically relevant parameter used to diagnose parathyroid adenomas intraoperatively. We evaluated the accuracy of a formula to estimate parathyroid weight on preoperative 4D-CT.
MATERIALS AND METHODS: A single-institution retrospective study was performed in patients with primary hyperparathyroidism who underwent 4D-CT between January 2013 and December 2014 with subsequent parathyroidectomy and surgical cure. All patients had correct localization of a solitary parathyroid adenoma. The longest 3 dimensions of all identified parathyroid glands were measured on CT, and weight was estimated using the formula: weight4D-CT (mg) = 1 mg/mm3 × Length (mm) × Width (mm) × Height (mm) × π/6. We correlated weight4D-CT with pathology specimen weight (weightpathology). Using receiver operating characteristic analysis, we estimated the performance of weight4D-CT to discriminate a parathyroid adenoma from normal glands on 4D-CT and determined the optimal threshold based on the Youden index.
RESULTS: One hundred sixteen patients (85 women, 31 men) were evaluated. Weight4D-CT was shown to be strongly correlated with weightpathology as demonstrated by Spearman ρ = 0.73 (P < .01), concordance correlation coefficient = 0.92 (95% CI, 0.89–0.94), and Cronbach α = 0.96. The performance of weight4D-CT for the diagnosis of parathyroid adenoma was excellent, with an area under the curve of 0.955 (95% CI, 0.925–0.985; P < .001). Based on the Youden index, the optimal threshold was >50 mg, with a sensitivity of 96.7% and a specificity of 95.7%.
CONCLUSIONS: Radiologists can accurately estimate parathyroid adenoma weight on 4D-CT. This metric is highly correlated with pathologic weight, and a threshold cutoff of >50 mg can be used to distinguish parathyroid adenoma from normal glands.
ABBREVIATIONS:
- AUC
- area under the curve
- IQR
- interquartile range
- ROC
- receiver operating characteristic
Parathyroid 4D-CT is a relatively new imaging technique for preoperative localization of parathyroid adenomas in patients with primary hyperparathyroidism. First reported by Rodgers et al,1 in 2006, 4D-CT has increasingly gained acceptance, most commonly as a second-line approach to the traditional methods of nuclear scintigraphy and sonography. It has also demonstrated the potential to be used as a first-line parathyroid imaging technique.2 Studies have demonstrated a relatively high sensitivity of 4D-CT for localization of parathyroid adenomas and hyperplasia, with reported sensitivities ranging from 76% to 88%1,3,4 and superior localization compared with sestamibi and sonography.4⇓-6 Accurate preoperative imaging allows surgeons to appropriately select patients for minimally invasive parathyroidectomy by localizing a single parathyroid adenoma and excluding multigland disease.2
While identification of parathyroid adenomas by 4D-CT relies on their characteristic differential enhancement across time, the classic enhancement pattern is observed in only a minority of adenomas (20%).7 In the setting of variable enhancement patterns, gland size may serve as an additional 4D-CT parameter for identifying adenomas preoperatively, particularly in distinguishing normal from abnormal glands. This is in line with the intraoperative approach of the parathyroid surgeon, because weight and size are the most frequently used criteria for identifying abnormal glands, with additional intraoperative features including shape, consistency, and histologic features.8 The weight of the pathologic specimen is considered to be the best measure of gland size, as opposed to gland length, due to the variable shape of parathyroid glands.9 Normal parathyroid glands generally weigh 20–40 mg, with glands of >60 mg usually considered abnormal by pathologists and surgeons.8,10
The high spatial resolution of 4D-CT allows delineation of adenomas from adjacent structures, which in theory, would allow an accurate estimate of adenoma size. While a pathologic size threshold may be inferred to 4D-CT, several factors may influence such estimates, such as adenoma shape, location, and relationship to and mass effect from adjacent structures, such as an enlarged multinodular thyroid gland or esophagus. These potential confounders do not affect the actual weight of the pathologic specimen following surgical resection. Studies in lung cancer and renal cell carcinoma correlating tumor sizes as measured on CT scans with pathologic specimens have shown poor correlation, with statistically significant differences in measured size on CT compared with pathologic size.11,12
Differential contrast enhancement remains the dominant factor in the detection of parathyroid adenomas, but a potential role for estimated adenoma weight by preoperative 4D-CT has not been evaluated. The size cutoff on 4D-CT to distinguish a normal gland from an adenomatous gland remains unknown. The aims of our study were to correlate the estimated gland weight of parathyroid adenoma on 4D-CT with the weight of the pathologically resected adenoma in patients with primary hyperparathyroidism and single-gland disease and to determine whether 4D-CT can define a threshold weight that distinguishes a normal gland from an adenomatous gland. We hypothesized that estimating weight on 4D-CT using a predefined formula will have a strong correlation with pathologic weight, and a threshold CT weight cutoff can differentiate adenomas from normal glands.
MATERIALS AND METHODS
Patients
A retrospective review was conducted of 250 consecutive patients with biochemical evidence of primary hyperparathyroidism referred to our institution for preoperative imaging using a combined imaging protocol of 4D-CT and sestamibi SPECT/CT between January 2013 and December 2014. Of note, an analysis of the diagnostic performance of this combined imaging protocol has been previously published by our group,6 and there is some overlap of patients from that study with patients in this cohort. Figure 1 summarizes the study patient flow. Of 250 patients, 138 patients underwent parathyroidectomy with surgical cure, defined by a 50% drop in intraoperative parathyroid hormone levels into the normal range. In-traoperative parathyroid hormone monitoring has been shown to have excellent accuracy in predicting surgical cure and is recommended by the Endocrine Society Guidelines.13,14 Most patients (n = 90 patients) had intraoperative parathyroid hormone normalization within 10 minutes of adenoma resection. Twenty-two patients were excluded for the following reasons: Eighteen patients had multigland disease, and 4 patients had nonlocalization or incorrect localization on 4D-CT. The remaining 116 patients with solitary adenoma correctly localized on 4D-CT were used for this analysis.
Study flowchart with inclusion and exclusion criteria. PHPT indicates primary hyperparathyroidism.
The study did not include patients in whom 4D-CT did not correctly localize the culprit solitary adenoma (3.3%, n = 4/120) with a mean adenoma pathologic weight of 136 mg. The reason for not including these individuals is that this was a correlation analysis of weight estimated on 4D-CT and pathologic specimen weight. The intention of the study was also to discriminate between the weights of normal and abnormal glands on 4D-CT, thus requiring inclusion only of those individuals with correct localization. This study was Health Insurance Portability and Accountability Act–compliant and approved by the institutional review board at Columbia University Medical Center.
4D-CT Imaging Protocol
The imaging protocol consisted of a combined sestamibi SPECT and 4D-CT consecutively acquired in a single setting previously published by our group.6 In brief, the protocol consists of a dual-phase technetium Tc99m sestamibi SPECT, followed by a 4D-CT performed immediately after acquisition of the delayed phase sestamibi SPECT/CT. For the purposes of this study, only the 4D-CT protocol will be discussed. All imaging was performed with a Symbia T 16-slice SPECT/CT scanner (Siemens).
The 4D-CT protocol consisted of helical scans acquired in noncontrast, arterial, and delayed (venous) phases at predetermined times. For all phases, scanning parameters were the following: 130 kV(peak), 120–300 mA by automatic exposure control, 0.6-second rotation time, 0.8 pitch, and 1.0-mm detector configuration with a beam width of 10 mm. Coronal and sagittal reformats were reconstructed with 1-mm section thickness. The FOV extended from the level of the mandibular angle to the carina. Following the noncontrast CT acquisition, iodinated contrast (iohexol, Omnipaque 350; GE Healthcare) was injected at 4 mL per second with a total dose of 75 mL. The arterial phase CT was acquired 30 seconds after the beginning of contrast infusion, and the delayed phase CT was acquired 30 seconds after the arterial phase acquisition.
Parathyroid Adenoma Localization and CT Estimated Weight
4D-CT images were interpreted for preoperative localization by 2 radiologists with dual board certification in radiology and nuclear medicine. 4D-CT images were reviewed on a PACS workstation. Parathyroid adenomas and normal parathyroid glands were identified using location and temporal contrast enhancement on serial phases. Following identification, adenoma volume was estimated in cubic millimeters using the formula for ellipsoid volume (V):
V4D-CT (mm3) = L (mm) × W (mm) × H (mm) × π/6.
Using the arterial phase CT, dimensions for length (L) and width (W) were measured on the axial plane in the longest perpendicular dimension, while height (H) was measured on either the coronal or sagittal plane, depending on the plane on which the adenomas were best visualized. Then, adenoma weight was estimated in milligrams by converting volume to weight. To this end, we assumed that all adenomas had the density of water (1 mg/mm3) and used the formula, weight (W) = density × volume (V).
W4D-CT (mg) = 1mg/mm3 × Vadenoma (mm3).
Therefore, the formula used for parathyroid weight on 4D-CT (weight4D-CT) was
Weight4D-CT (mg) = 1 mg/mm3 × L (mm) × W (mm) × H (mm) × π/6.
Estimated weights of suspected parathyroid adenomas and any additional visualized normal parathyroid glands were measured and described in the original radiology report, per standard clinical practice at our institution. Both interpreting radiologists were trained on this method for estimating weight on CT. Of the 116 patients, 75 patients had 4D-CT scans that identified and estimated CT weights of 3 normal parathyroid glands in addition to the abnormal parathyroid adenoma, 30 patients had 2 normal parathyroid glands identified, 6 patients had 1 normal parathyroid gland identified, and 5 patients had no normal parathyroid glands identified. In total, 407 parathyroid glands (normal, n = 291, and adenomas, n = 116) were identified in 116 patients.
Pathology
Surgically resected parathyroid glands were placed in formalin for fixation. Extracapsular fat was dissected off the surgical specimen, when appropriate. The weight (milligrams) of the surgical specimen was recorded. Pathologic examination of the specimen was performed to confirm parathyroid adenoma.
Statistical Analysis
The radiology, operative, and pathology reports were retrieved from the Electronic Medical Record. CT-estimated weight and pathologic weight were recorded on the basis of original radiology and pathology reports, respectively, and no re-interpretation or repeat measurements were performed.
Data were assessed for normality by visual inspection of the histograms. Normally distributed data are presented as mean ± SD, while nonparametric data are presented as median and interquartile range (Q25–Q75). We used 3 metrics to compare CT-estimated weight and pathologic weight measurements: the Spearman correlation coefficient, the concordance correlation coefficient, and the Cronbach α.15
To determine the weight cutoff for parathyroid adenomas on 4D-CT, we constructed a receiver operating characteristic (ROC) curve with the normal parathyroid gland or parathyroid adenoma as the state variable and CT-estimated weight as the test variable, and the area under the ROC curve (AUC) and 95% confidence interval were estimated. Because multiple gland weights were included per patient, ROC analysis incorporating clustering of observations within a patient was performed. The Youden index (sensitivity + specificity − 1) was calculated to determine the optimal threshold for CT-estimated weight to discriminate a parathyroid adenoma from a normal gland. Using cross-validation analysis, we randomly selected 80% of the sample for the training set and applied ROC curve analysis to obtain the optimal threshold. We then tested the threshold against the remaining 20% of the sample for its performance. As an ancillary study, we also evaluated the performance of parathyroid length (millimeters) using the longest single dimension. All statistical tests were 2-tailed, and P < .05 was considered significant. All analyses were performed with SPSS 23.0 for Windows (IBM).
RESULTS
Patient Characteristics
Table 1 summarizes the study patient characteristics and parathyroid gland weights. The study population (n = 116) was predominantly female (n = 85, 73.3%), with a mean age of 63 ± 14 years. Median serum parathyroid hormone and calcium levels were 101 pg/mL (interquartile range [IQR] = 67.0–135 pg/mL) and 10.8 mg/dL (IQR = 10.4–11.4 mg/dL), respectively. Parathyroid adenomas (n = 116) had a median CT weight of 310 mg (IQR = 145–536 mg) and pathologic weight of 500 mg (IQR = 238–800 mg), with a mean difference of 130 mg between CT and pathologic weights. Normal parathyroid glands (n = 291) had a median CT weight of 18 mg (IQR = 12–24 mg). Figure 2 demonstrates a boxplot of CT weights of parathyroid adenomas compared with normal glands.
Boxplot of weight4D-CT in milligrams of parathyroid adenomas compared with normal parathyroid glands.
Patient demographics, baseline characteristics, and parathyroid gland weightsa
Correlation between Weight4D-CT and Weightpathology
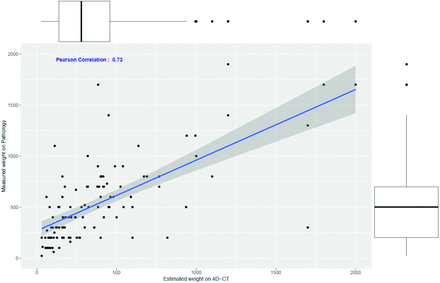
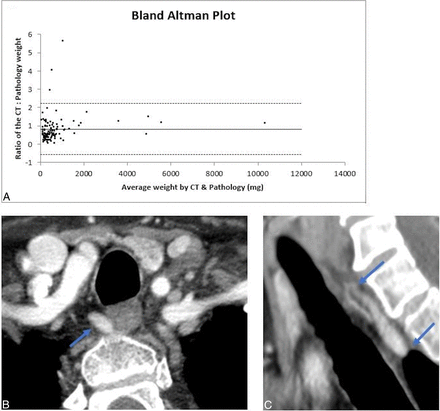
CT-estimated weight had a strong positive correlation with the pathologic adenoma weight (r = 0.73, P < .01) (Fig 3). The concordance correlation coefficient between the 2 measurements was 0.92 (95% CI, 0.89–0.94), showing moderate agreement, and the Cronbach α was measured as 0.96, demonstrating excellent reliability. A Bland-Altman plot showed good agreement between the estimated weight by CT and the measured pathologic weight (Fig 4). There was a negative systemic bias, with the CT-estimated weight underestimating the pathologic weight, with a mean ratio of weight4D-CT to weightpathology of 0.83. Thus, the pathologic weight can be predicted by the formula:
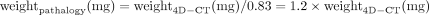
Scatterplot showing the relationship between weight4D-CT in milligrams of the parathyroid adenoma (x-axis) and the measured pathologic weight of the resected parathyroid adenoma, weightpathology (y-axis). The blue line indicates the Pearson correlation (r = 0.73, P < .01).
Correlation between Weight4D-CT and Weightpathology. A, Bland-Altman plot. The solid line represents the mean ratio (0.83), and the dashed lines represent the lower and upper 95% limits of agreement at 0.56 and −2.22, respectively. Representative imaging case of 63-year-old woman with right upper parathyroid adenoma. Axial (B) and Sagittal (C) arterial phase images from preoperative 4D-CT demonstrate a teardrop-shaped right upper parathyroid adenoma in the right tracheoesophageal groove measuring 10 × 5 × 19 mm (transverse × anterior-posterior × cranio-caudal), with weight4D-CT of 475 mg. Weightpathology was 530 mg with CT: pathologic weight ratio of 0.90.
Diagnostic Accuracy of Weight4D-CT for the Diagnosis of Parathyroid Adenoma
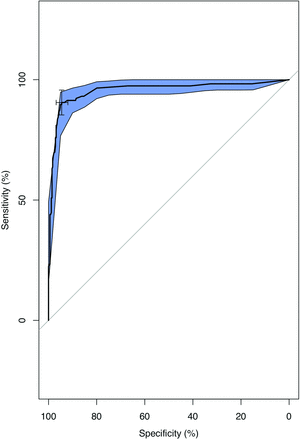
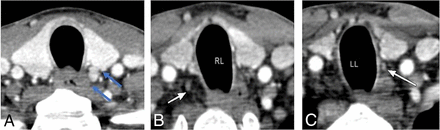
CT-estimated weight (Fig 5) reached an AUC of 0.955 (95% CI, 0.92–0.985; P < .001) for the prediction of parathyroid adenoma. On the basis of the Youden index, a threshold weight >50 mg was the most optimal in discriminating between a parathyroid adenoma and normal parathyroid gland, with a sensitivity of 96.7% and a specificity of 95.7%. We tested the threshold cutoff of 50 mg in the validation set, with a similar sensitivity of 100% and a specificity of 96.6%. Of note, the performance characteristics were similar across a range of thresholds from 40 to 80 mg (Table 2). For example, a threshold of >40 mg had a sensitivity of 96.4% and a specificity of 92.3%, a threshold of >60 mg had a sensitivity of 94.4% and a specificity of 96.2%, and a threshold of >80 mg had a sensitivity of 90.0% and a specificity of 97.4%. Figure 6 demonstrates a case of a patient with a small adenoma and additional identified normal glands.
ROC curve showing the diagnostic performance of CT-estimated parathyroid weight in discriminating parathyroid adenoma from the normal parathyroid gland, with confidence intervals (blue) (AUC = 0.955; 95 CI, 0.925−0.985; P < .001).
A 61-year-old woman with left upper parathyroid adenoma. A, Axial arterial phase CT image demonstrates a left upper parathyroid (blue arrows) with CT-estimated weight of 70 mg. Additional normal right lower (24 mg) (B) and left lower (18 mg) (C) parathyroid glands are identified (white arrows). RL indicates right lower; and LL, left lower.
Performance characteristics for various CT-estimated weight thresholds
Using longest dimension of a parathyroid gland on CT, the AUC was 0.981 (95% CI, 0.969–0.993) for prediction of parathyroid adenoma (On-line Figure), with an optimal threshold length of >6.5 mm based on the Youden index, yielding a sensitivity of 94.8% and a specificity of 95.9%.
DISCUSSION
We evaluated the use of preoperative 4D-CT in estimating parathyroid adenoma weight with a predefined formula of CT-derived measurements compared with the pathologic weight in patients with primary hyperparathyroidism and solitary parathyroid adenomas. Our results showed that CT-estimated weight and pathologic weight had a strong positive correlation, moderate agreement, and excellent reliability. These findings demonstrate that 4D-CT can closely predict the parathyroid adenoma weight at resection using a simple formula. Because CT underestimates the pathologic weight by a mean ratio of 0.83, we propose using a refined formula to predict pathologic weight: weightpathology = 1.2 mg/mm3 × L (mm) × W (mm) × H (mm) × π/6, which can be simplified further to weightpathology = 0.63 mg/mm3 × L (mm) × W (mm) × H (mm). In clinical practice, this formula could be easily implemented using an automated calculator or Excel (Microsoft) spreadsheet and the estimated parathyroid weight reported in the radiology report. Adenoma weight is a critical factor in the surgeon's intraoperative approach and decision-making, and providing an accurate preoperative weight estimate allows the surgeon to more effectively plan the operation.
Our study also determined an optimal CT size cutoff of >50 mg to distinguish a parathyroid adenoma from a normal gland, with high diagnostic performance. This suggests that CT-estimated weight can be used to distinguish solitary parathyroid adenomas from normal parathyroid glands and is particularly important in localizing smaller adenomas or when differential CT contrast enhancement is equivocal or unreliable, occurring not infrequently due to poor contrast bolus timing or streak artifacts from contrast injection or the patient's shoulders. Because accurate localization of the abnormal parathyroid adenoma is critical for a minimally invasive parathyroidectomy, a low false-negative rate and thus a higher sensitivity may be more favorable to surgeons. For our refined formula, we propose a corrected CT size cutoff of >60 mg (1.2 × 50 mg). This finding is concordant with the pathologic weight cutoff of >60 mg used by pathologists and surgeons8,10 and demonstrates that similar thresholds were still obtained despite using differing methodologies of preoperative CT scans and autopsy studies. In addition, the CT-estimated weights of normal glands in our study are similar to those reported in prior autopsy studies.16,17
There are several potential reasons for the slight underestimation of adenoma weight on 4D-CT. Given the location of the parathyroid glands, parathyroid adenomas are in close proximity to other anatomic structures, including the thyroid, thymus, carotid artery, jugular vein, and neck muscles, which can result in mass effect and compression of parathyroid adenomas. For example, compression of a parathyroid adenoma can be seen in cases of concomitant enlarged multinodular thyroid goiter or when parathyroid adenomas are very large and confined by adjacent structures. Second, surgical specimens may include adjacent fat and thymic gland, which may lead to a higher measured pathologic weight. Third, our method of estimating weight on CT is based on assumptions that parathyroid adenomas are ellipsoid and have a density equal to that of water. Differences in adenoma shape (teardrop, cylindric, and so forth) would, therefore, affect the CT-estimated weight. Also, the true density of parathyroid tissue is slightly greater than that of water, with density in the range of 1.049–1.069 mg/mm.3,18 The amount of stromal fat in the parathyroid gland may also affect the density of the gland.19 It is plausible that adenomatous changes in the parathyroid gland decrease the fat content and render the parathyroid adenoma denser, another factor for underestimation of the pathologic weight by CT-based weight estimates. Only 1 prior study has evaluated the correlation of CT-estimated weight and pathologic weight and showed a positive correlation with a Pearson correlation coefficient of 0.96.20 We also found a positive correlation with a Spearman correlation coefficient of 0.73. The Spearman correlation was used in our study because of the skewed distribution in our dataset.
Prior studies have shown that parathyroid adenoma size can affect the localization accuracy of sestamibi,21,22 with lower accuracy in smaller glands. However, the effect of parathyroid adenoma size on the diagnostic accuracy of 4D-CT is not as well-established, with few studies in the literature. Day et al23 evaluated 4D-CT in patients with negative findings on sonography or sestamibi scans and found that the mean pathologic weight of glands successfully localized by 4D-CT was 404 mg compared with 259 mg for those not localized by 4D-CT. Similarly, Galvin et al3 showed that the mean weight of glands missed on 4D-CT was 0.3 g compared with 0.6 g in detected glands, but there was no statistical difference (P = .15). In our cohort, the mean pathologic weight of adenomas not successfully localized on 4D-CT was 136 mg; however, this estimate is based on only 4 patients. While prior studies were based on postoperative pathologic weight, our study allows extrapolation of these findings to preoperative CT-estimated weight by demonstrating a strong positive correlation between CT-estimated weight and pathologic weight. A preoperative 4D-CT size evaluation by Sho et al24 demonstrated that abnormal parathyroid glands missed on 4D-CT were smaller, with mean of 8.6 mm versus 12.4 mm (P < .001), and parathyroid glands of ≥10 mm had higher chances of nonlocalization on 4D-CT (odds ratio = 4.37; 95% CI, 2.24–8.54). We found that a parathyroid gland length cutoff of >6.5 mm for the longest dimension could potentially be used to differentiate adenomas from normal glands; however, the longest dimension may not be the most accurate measurement of parathyroid size, given the differences in parathyroid gland shape and morphology.
There are several limitations in our study. First, our CT-estimated weights were calculated assuming an ellipsoid volume and density of water. More accurate volume assessments can be obtained through manual CT segmentation of the adenoma; however, this method would not be ideal in standard clinical workflow. Second, we included only patients with parathyroid adenomas correctly localized by 4D-CT with subsequent surgical cure, which may influence our results. Only 4 patients had unsuccessful localization on 4D-CT, and this is probably due to the high diagnostic accuracy of 4D-CT. Last, we also limited the study to include only patients with solitary parathyroid adenomas; thus, whether these results can be applied to patients with multigland disease remains to be studied.
CONCLUSIONS
This study demonstrates that using a formula to estimate parathyroid adenoma weight on 4D-CT is valid and reproducible, and a CT weight cutoff of >50 mg can be used to distinguish a parathyroid adenoma from normal glands. Our findings can be applied in routine clinical practice to help radiologists improve interpretation and reporting of 4D-CT scans and guide surgeons in preoperative planning. Future directions include evaluating this formula in patients with multigland disease.
References
- Received February 17, 2020.
- Accepted after revision June 2, 2020.
- © 2020 by American Journal of Neuroradiology