Abstract
SUMMARY: [18F]FDG-PET is a widely used technique for specific evaluation of disease and treatment response in oncology. However, the principles behind [18F]FDG-PET imaging allow a wide-ranging array of benign and malignant pathologies to be identified on both initial and routine surveillance imaging. This is important for clinicians and radiologists, alike, in that effective and accurate evaluation of malignancy and metastatic disease, specifically involving the spine and central nervous system, is crucial. In this article, we review the normal and posttherapy appearance of the spine on [18F]FDG-PET, the various types and patterns of metastatic disease that involve the spine and spinal cord, and, finally, important spinal pathologies that may mimic malignancy on [18F]FDG-PET.
ABBREVIATIONS:
- G-CSF
- granulocyte colony-stimulating factors
- ISCM
- intramedullary spinal cord metastasis
- SUV
- standard uptake value
- SUVmax
- maximum standard uptake value
Since its inception in the mid-1970s, [18F]FDG-PET has grown into a multifaceted tool with applications not only in cancer imaging but in neurologic disorders, infection, inflammation, and cardiac imaging.1 As one of the most quantitative imaging techniques available for assessing metastatic disease, [18F]FDG-PET/CT has become an essential imaging tool in the diagnosis, staging, and management of cancer and cancer-related disease during the past two decades.1 Although metastatic disease can occur anywhere, the spine is of particular importance, not only because it is the third most frequent site of distant metastatic disease but also because many nonmalignant processes, some of which can appear nearly identical to metastatic foci on [18F]FDG-PET, are frequently identified involving the spine during the course of a patient’s routine oncologic work-up.2
While MR imaging is the most crucial imaging technique used to assess spinal metastatic disease, various metastatic disease patterns have been demonstrated on [18F]FDG-PET, which can help in disease localization and assessment.2,3 Understanding both the benefits and pitfalls of [18F]FDG-PET in evaluating the spine is important, given the frequent use of PET and PET/CT in both oncologic work-up and surveillance. This review will discuss general [18F]FDG-PET and, most important, nonmetastatic pitfalls that may appear similar on standard [18F]FDG-PET.
Normal Distribution of [18F]FDG in the Spine
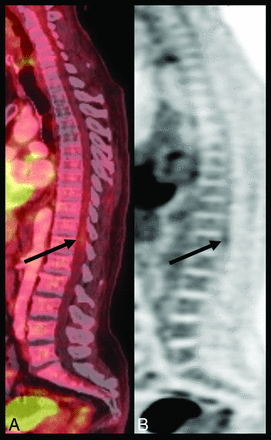
In the assessment for metastatic disease in the spine, recognition of the normal or physiologic appearance of [18F]FDG-PET is essential. Because [18F]FDG uptake in PET reflects tissue levels of cellular glucose metabolism, normal anatomic structures in the spine can demonstrate variable degrees of hypermetabolic uptake. Specifically, relative increases in physiologic [18F]FDG uptake have been demonstrated in the spinal cord at the T11 and T12 levels and, to a lesser degree, at the C4 level (Fig 1).4⇓-6 Additionally, slight relative physiologic uptake within the cord has also been noted at the level of the atlas.7 While not definitively explained, it is theorized that the increased uptake in the lower thoracic cord is due to inadequate clearance of the radiotracer from the artery of Adamkiewicz, which originates from the aorta between T9 and T11, and/or due to the relative increased cross-sectional area of the spinal cord at the midcervical and lower thoracic levels with an associated increased ratio of gray matter.4,5,7
A 59-year-old man with lung cancer without metastatic disease. Sagittal fused (A) and AC PET (B) images demonstrate physiologic [18F]FDG uptake throughout the spine as well as focal physiologic [18F]FDG spinal cord uptake at T11–T12 (arrows). Absent uptake in the midthoracic spine is related to previous radiation therapy. Fused indicates fused PET and CT image; AC, attenuation-corrected.
Relative changes in physiologic uptake can also be noted within the vertebral bodies, with background marrow uptake typically having a maximum standard uptake value (SUVmax) of <3.8 Peak physiologic radiotracer uptake has been noted within the lower thoracic vertebral bodies, typically between T8 and T11, though standard uptake values (SUVs) are usually below those of the liver.8,9 Additionally, although subtle, SUVs typically demonstrate a gradual decrease both cranially and caudally.6,9 Because this increased uptake often appears as focal areas within the marrow and can be misleading on axial images, it is important to correlate with the sagittal and coronal planes. Because [18F]FDG uptake is dependent on active hematopoietic marrow–red marrow, studies have shown a gradual decrease in osseous [18F]FDG uptake with increasing age as red marrow is replaced by yellow marrow.8
Posttherapy Changes of the Spine
Many cancer therapies play an important role in the oncologic application of [18F]FDG-PET, with two of the most common being granulocyte colony-stimulating factors (G-CSF) and radiation therapy. G-CSF is a glycoprotein hormone used to treat chemotherapy-induced neutropenia and reduce infection severity by stimulating hematopoietic progenitors.10 Diffusely increased, homogeneous radiotracer uptake is identified throughout the bone marrow both during and after G-CSF administration in up to 87% of patients.10,11 Given this diffuse marrow uptake, both bone metastases and benign bone lesions may be obscured or appear as photopenic defects due to the relative hyperplastic bone marrow (Fig 2).11 Although the optimal timeframe for follow-up PET/CT in the setting of G-CSF therapy has not been determined, studies have shown that bone marrow [18F]FDG uptake can remain elevated for up to 1 month after administration of G-CSF, with return to plateau times ranging from 10 days to 1 month.11,12
A 62-year-old woman with breast cancer. Sagittal CT (A), AC PET (B), and fused (C) images demonstrate a sclerotic (asterisk) and photopenic region (solid arrows) in the L1 vertebral body consistent with a site of treated metastasis. Note the diffusely increased radiotracer uptake through the remaining axial skeleton, which obscures multilevel osseous metastases seen on CT. Fused indicates fused PET and CT image; AC, attenuation-corrected.
Radiation therapy can also have considerable effects on normal tissue, especially hematopoietic bone marrow. Specifically, radiation therapy can cause immediate avid [18F]FDG uptake due to local postradiation inflammation.10 Therefore, [18F]FDG-PET is typically performed 8–12 weeks after completion of radiation therapy for better assessment of the treatment response.13 In the subacute and chronic stages after radiation therapy, treated areas of bone marrow typically appear as photopenic regions, matching the geographic radiation field (Fig 3).10,11 Some patients have experienced [18F]FDG uptake in irradiated bone marrow gradually decreasing below baseline levels as early as 2–8 days after therapy.14
A 48-year-old man with B-cell lymphoma of the gastric fundus after radiation therapy. Sagittal CT (A), AC PET (B), and fused (C) images demonstrate a large photopenic segment (bracket) in the radiation field. Note the absence of a correlative abnormality on CT in this region. Fused indicates fused PET and CT image; AC, attenuation-corrected.
Metastatic Disease of the Spine
The spine is the third most common site for distant metastatic disease after the lung and liver and is the most common site for osseous metastases, with approximately 50%–70% of patients with systemic cancer having spinal involvement.2 Involvement of the spine in the setting of cancer can be divided into distant metastases, either through hematogenous or lymphatic spread or by extension from surrounding tissues, including by local invasion or perineural spread.2 While the conventional oncologic work-up for spinal metastatic disease involves detailed MR imaging evaluation, [18F]FDG-PET is often performed first during the initial staging and can offer valuable information, given its reliance on metabolic activity.3
Metastatic Disease
Osseous.
The spine is the third most common site of metastatic disease, following the lung and liver, with lung, breast, and prostate cancer the most commonly identified primary sites.15 The thoracic spine is the most commonly involved vertebral level, possibly due to the relatively increased degree of bone marrow volume to receive hematogenously spread metastatic deposits.2,16 [18F]FDG-PET is an important tool for the diagnosis of early osseous metastatic disease because increased glucose metabolism in neoplastic cells can become evident in even the earliest cases of bone marrow infiltration.15,17 [18F]FDG-PET can demonstrate in-creased radiotracer uptake regardless of lesion type, either osteolytic or osteoblastic, though due to a multitude of factors including biochemical activity of these lesions, the degree of [18F]FDG uptake can be variable (Fig 4).15,18
A 66-year-old woman with breast cancer. Sagittal CT (A), fused (B), and AC PET (C) images demonstrate extensive osseous metastases throughout the spine with a number of lesions demonstrating varying degrees of increased uptake (arrows). Heterogeneous radiotracer uptake is due to posttreatment changes. Absent radiotracer uptake suggests treated disease including in sclerotic vertebral bodies. This case demonstrates the superiority of PET/CT over conventional imaging in demonstrating a response to therapy. Fused indicates fused PET and CT image; AC, attenuation-corrected.
PET/CT is superior to CT for the evaluation of treatment response, though imaging considerations in treatment response between PET/CT and MR imaging are more complicated, because specific disease processes may alter which is the most accurate method. While there are morphologic MR imaging findings indicative of both treatment response (eg, disappearance of focal lesions, decreased size/number of lesions) and disease progression (eg, increased number/size of lesions or evolution from focal to diffuse neoplastic infiltration), problems such as arrested resolution of abnormalities despite effective therapy that are thought to be due to bone sclerosis, marrow fibrosis, or necrosis as well as difficulty in evaluating disease activity on a scarred background and differences in MR imaging techniques limit morphologic assessment.19 Advanced MR imaging techniques such as perfusion and diffusion imaging can be used to supplement morphologic assessment through their assessment of tumor perfusion/permeability and cellular density/integrity, respectively.20 Like MR imaging, [18F]FDG-PET also has issues when assessing only FDG-avid tumors as well as in the setting of flare reactions after G-CSF administration. Additionally, the choice of imaging technique, notably with the development of PET/MR imaging, should depend on the most accurate way to assess the primary lesion, especially in cases of osseous metastases.20
Epidural.
With an incidence of up to 5%–10%, epidural metastatic disease can be seen in up to 40% of patients with pre-existing nonspinal osseous metastases.21 Prostate, breast, and lung cancer account for the most cases of epidural involvement.21 Because thoracic spine involvement is most common, in approximately 60% of cases, epidural disease has the greatest likelihood of producing spinal cord injury.15,21,22 Epidural involvement can result from hematogenous and lymphatic dissemination or by contiguous extension from an adjacent vertebral body or through a neuroforamen.3,15 While MR imaging is superior to CT for evaluating epidural involvement, superimposed PET does improve the sensitivity of CT for detection, particularly when there is no associated adjacent osseous destruction (Fig 5).3 However, conventional PET alone is too limited in its spatial resolution, at about 4–5 mm, to differentiate epidural from intradural disease, and while fused CT imaging can improve its sensitivity, suggested spinal canal disease should prompt analysis with MR imaging.23
An 80-year-old woman with mucosa-associated lymphatic tissue lymphoma. Axial fused (A), sagittal fused (B), and sagittal T2-weighted MR images (C) demonstrate an intensely hypermetabolic focus within the spinal column at T7–T8 (dashed arrow), which corresponds to a T2-isointense posterior epidural mass (solid arrow), which was found to be biopsy-proved metastatic lymphoma. Fused indicates fused PET and CT image.
Intramedullary.
Intramedullary spinal cord metastasis (ISCM) is one of the rare forms of systemic metastatic disease, comprising between 1% and 3% of all patients with metastatic disease and up to 9% of those with central nervous system involvement.3 Approximately 50% of ISCMs arise from a primary lung cancer, followed by breast cancer as the second most common source.24,25 Up to one-third of these patients were shown to have concurrent brain metastases, and up to one-fourth had additional leptomeningeal carcinomatosis.25
Numerous studies have demonstrated an increased prevalence of thoracic spinal cord involvement.24 These lesions tend to show SUV uptake greater than the mediastinal blood pool, with one study showing an average SUVmax of 6.7.26,27 The morphology of [18F]FDG uptake on PET tends to demonstrate round hypermetabolic foci in most cases (Fig 6). In addition, most MR imaging–visible ISCMs tend to be seen on PET as well.3 MR imaging features that correlate with visibility on PET include a larger lesion enhancement area, a larger extent of T2 signal abnormality, and an increased ratio of T2 signal abnormality to contrast enhancement.26
A 54-year-old woman with breast cancer. Sagittal AC (A) and fused (B) images show a linear segment of hypermetabolic activity (asterisk) in the thoracolumbar spinal column. This correlates with multiple enhancing intramedullary metastatic lesions (arrows) on corresponding sagittal T1-weighted postcontrast MR imaging (C). Fused indicates fused PET and CT image; AC, attenuation-corrected.
Leptomeningeal.
Leptomeningeal disease or leptomeningeal carcinomatosis involves the presence of metastatic cells within the subarachnoid space of the brain and spinal cord. Etiologies range from breast, small-cell lung cancer, melanoma, leukemia, and head and neck cancers, with up to 2%–5% of patients with breast cancer developing leptomeningeal disease.3,28,29 The pathogenesis is thought to occur by either hematogenous spread, extension through perivascular or perineural lymphatics, or direct extension from adjacent tumor.2,3 Although leptomeningeal disease is often undiagnosed or clinically silent, up to 98% of patients are symptomatic at the time of diagnosis.3,30
Leptomeningeal disease shows variable radiotracer uptake on [18F]FDG-PET, ranging from 2.8 to 11.1 SUVmax in one study.30 The uptake pattern appears similar to the respective pattern of contrast enhancement on MR imaging (Fig 7). A classic example, the “bottle brush sign,” demonstrates FDG-avid disease within the lumbosacral spinal canal, extending through the sacral neural foramina.3 One limitation, however, is that patients with only thin linear or fine multinodular enhancement patterns on MR imaging demonstrated increased false-negative findings on PET studies.30 This is because most leptomeningeal disease is below the spatial resolution threshold of [18F]FDG-PET.3
A 61-year-old man with chronic lymphocytic leukemia. Sagittal fused (A), AC (B), and postcontrast T1-weighted MR images (C) demonstrate a hypermetabolic focus within the anterior thoracic spinal canal (dashed white arrow) corresponding to a solid, enhancing intradural extramedullary lesion (solid white arrow), which was found to be a schwannoma. There is additional subtle hypermetabolic uptake predominantly along the inferior thoracic cord (dashed circle), which demonstrates a “sugar-coating” pattern of enhancement on MR imaging, consistent with leptomeningeal spread of disease. Fused indicates fused PET and CT image; AC, attenuation-corrected.
Direct Extension
Perineural.
Perineural spread of malignancy, an under-recognized route of disease spread, describes the process of neoplastic dissemination along a nerve. This spread occurs along the pathway of least resistance, which is between the neural axon and surrounding perineural layer.31,32 The incidence of perineural tumor spread ranges from 2.5% to 5%, with head and neck malignancies the most common cause.32,33
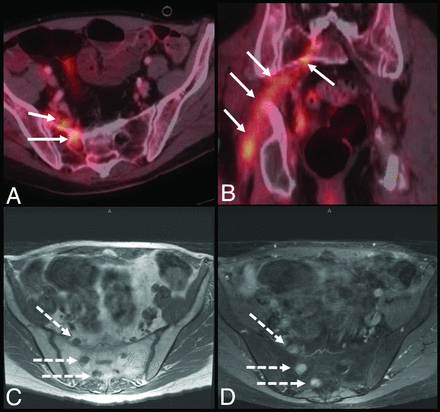
[18F]FDG-PET demonstrates a sensitivity and specificity of 83% and 90%, respectively, in the detection of perineural tumor spread.34 [18F]FDG-avid perineural lesions demonstrate linear or curvilinear increased uptake along the associated nerve in a discontinuous or nodular pattern, similar to MR imaging enhancement patterns (Fig 8).35 Perineural [18F]FDG uptake can be subtle, given the low spatial resolution of [18F]FDG-PET.35 Additionally, apart from the axial plane, one must use sagittal and coronal PET/CT images as well as MIP images for proper assessment. Limited analysis has shown that the mean SUVmax in patients with perineural metastatic spread is 7.1 (SD, 3.7).36 Secondary findings associated with perineural spread relate to eventual denervation and associated muscle atrophy, with [18F]FDG-PET demonstrating increased uptake within the affected muscle in the acute phase followed by normalization in later stages and eventual decreased uptake in chronic atrophy.35 False-positives with [18F]FDG-PET can be seen in cases of inflammation from prior radiation or surgery, especially within 1 month of surgery, with variable physiologic uptake in the adjacent musculature and lymphoid tissue as well as due to coregistration artifacts during PET and CT fusion.31,35
A 62-year-old man with penile cancer. Axial and coronal fused (A and B) images demonstrate a long, nodular segment of radiotracer uptake within the right pelvis suspicious for perineural spread of metastases (white arrows). Corresponding axial T1-weighted precontrast and T1-weighted fat-saturated postcontrast MR imaging (C and D) show nodular thickening and enhancement (dashed arrows) along the right sacral nerve roots. Fused indicates fused PET and CT image.
Direct Invasion.
Direct invasion of tumor into the paraspinal soft tissues, vertebral bodies, and spinal canal is a frequent occurrence. Direct extension to the spinal column can be either from a primary site or a secondary site such as a local metastatic lymph node and is typically accompanied by a paraspinal soft-tissue mass, which is not seen with hematogenous metastases.2
Nonmetastatic Disease of the Spine
Trauma and Degeneration.
Commonly encountered nonmetastatic spinal pathologies can pose challenges in patients in oncology undergoing [18F]FDG-PET imaging. Specifically, traumatic injuries and age-related degenerative changes of the spine are two important areas of concern because osseous metastatic disease and fractures can present in a similar fashion.37 Sacral insufficiency fractures, in particular, can mimic pelvic osseous metastases; however, these tend to have more linear or H-shaped pattern of uptake compared with the nodular patterns seen with metastatic disease (Fig 9).11,38 A key differentiator is the transient nature of [18F]FDG uptake in traumatic fractures, occurring due to the acute local inflammatory state, with no considerable uptake generally identified after 2–3 months.11,39
An 87-year-old woman with lung cancer. Coronal CT (A), [18F]FDG-PET (B), and PET/CT (C) images at the sacroiliac level demonstrate bilateral linear lucencies through the sacral ala (white arrows), with corresponding linear radiotracer uptake (black arrows) compatible with insufficiency fractures.
Degenerative and inflammatory arthropathies of the spine can also show mild-to-intense [18F]FDG avidity. In these cases, the degree of uptake is not necessarily linear in relation to the appearance of the degeneration but rather related to the degree of active inflammation.11,37,40 These findings most commonly are found near the vertebral body endplates and facet joints and include formation of synovial cysts, subchondral cysts, and osteophytosis, which can be difficult to delineate from lytic and blastic osseous metastases (Fig 10).11,37 Within the posterior elements, Baastrup disease, characterized by inflammatory changes involving the interspinous bursa and sclerosis of the spinous processes, can demonstrate mild-to-moderate [18F]FDG uptake and mimic posterior element metastases.38
A 68-year-old woman with breast cancer. Axial (A) and coronal (C) CT and axial (B) and coronal (D) fused images demonstrate an intense focus of increased [18F]FDG uptake in the lumbar spine (dashed arrows) corresponding to a bulky osteophytic pseudoarthrosis on CT (solid arrows), which can mimic blastic osseous metastases. Fused indicates fused PET and CT image.
Primary Osseous Lesions.
Primary osseous pathology, while not always neoplastic, is commonly encountered on routine surveillance oncologic imaging. These lesions, notably multiple myeloma and hemangiomas, can mimic metastatic disease and are important considerations during the evaluation of osseous metastatic disease. Because myelomatous lesions are metabolically active, fused imaging with CT can demonstrate hypermetabolic lytic lesions, which can be easily confused with lytic metastases (Fig 11).41,42 Hemangiomas, on the other hand, typically present as incidental photopenic lesions on [18F]FDG-PET (Fig 11), though occasionally internal hemorrhage and subsequent inflammatory changes of a vertebral hemangioma can demonstrate hypermetabolism.43⇓-45
Upper row: A 50-year-old woman with multiple myeloma. Sagittal fused (A) and CT (B) images demonstrate foci of intensely increased FDG uptake (dashed arrows) in the thoracolumbar spine vertebral bodies, corresponding to lytic myelomatous lesions (solid arrows) on CT, which in the absence of a proper history, can appear as lytic osseous metastases. Lower row: A 62-year-old woman with breast cancer after recent chemotherapy. Sagittal fused image (C) demonstrates an incidental photopenic lesion (dashed arrow) in the posterior T6 vertebral body corresponding to a hemangioma on CT (solid arrow). Fused indicates fused PET and CT image.
Benign Neurogenic Lesions.
Both primary malignant neoplasms of the spinal cord (eg, astrocytoma, ependymoma) as well as benign neurogenic lesions such as schwannomas can also mimic metastatic disease on PET.31 Schwannomas, which are the most common of the peripheral nerve sheath tumors, demonstrate variable [18F]FDG uptake and, in the setting of known malignancy, can appear similar to perineural spread of tumor, especially ones that demonstrate mild uptake (Fig 12).46,47
A 74-year-old man with thyroid cancer. Axial T2-weighted MR imaging (A), fused (B), and AC (C) images demonstrate mild increased [18F]FDG uptake (dashed arrows) within the paraspinal region at the C3–C4 level, appearing as a rounded soft-tissue density with neuroforaminal widening/remodeling and intermediate T2 signal on MR imaging (solid arrow). Findings corresponded to a schwannoma, which, in the setting of known primary malignancy, can mimic perineural metastasis. Fused indicates fused PET and CT image; AC, attenuation-corrected.
Infection.
Given the overexpression of the glucose transport protein 1 subtype in macrophages, lymphocytes, and neutrophils, infectious processes can also demonstrate hypermetabolism on [18F]FDG-PET mimicking metastatic disease (Fig 13).11,48 Of particular note, tuberculous spondylitis can demonstrate multilevel subligamentous spread mimicking paravertebral lymphadenopathy in metastatic disease or lymphoma.49
A 70-year-old man with rectal cancer. Axial CT (A), fused (B), and AC (C) images demonstrate intense hypermetabolic uptake (dashed arrows) in the paraspinal musculature of the mid and lower thoracic spine, which corresponds to a hypodense region of phlegmon/developing abscess on CT (solid arrow). Fluid cultures were positive for methicillin-resistant Staphylococcus aureus. Fused indicates fused PET and CT image; AC, attenuation-corrected.
Additional Considerations
Because the spatial resolution of [18F]FDG-PET is limited compared with conventional imaging, true disease assessment can be considerably hindered by partial volume effects, in which [18F]FDG concentrations in adjacent tissues, below the reconstruction resolution, can underestimate true tumoral metabolic activity.50,51 In response, multiple partial volume correction methodologies are increasingly being developed to overcome this limitation, critical for the assessment of treatment response.
As calculation of total disease burden becomes of increasing clinical importance, alternatives in the method by which [18F]FDG-PET data are analyzed has been studied. Particularly, total metabolic tumor volume and total lesion glycolysis have become more beneficial than typical SUVs regarding true tumor burden, risk stratification, and outcomes.52,53 Of note, the calculation of total lesion glycolysis uses SUVmean, which, while affected by inter- and intraobserver variability, is less sensitive to image noise and reconstruction parameters and may make total lesion glycolysis more beneficial in assessing tumor burden compared with SUVmax.54⇓-56 Although the time-consuming nature of manual quantification and correction makes use of total lesion glycolysis impractical for routine clinical practice, advancements in quantification software may make this limitation a moot point.57,58
The potential applications of recently developed total-body PET imaging instruments have led to exciting advancements in clinical nuclear medicine and molecular imaging. With their increased axial FOV, these scanners use increased detection efficiency and scanner sensitivity to considerably improve the signal-to-noise ratio and temporal resolution, all while using a lower radiopharmaceutical dose, which can be specifically useful in determining the extent of disease in the spine and spinal cord.59,60 However, a major limitation for institutions outside of large research institutions remains the cost of these scanners, particularly the scintillation material, as well as data storage and processing concerns.61
CONCLUSIONS
Spinal involvement by malignancy, either by direct extension or distant metastases, is a relatively common occurrence in the work-up and management of patients with cancer. While CT and MR imaging play important roles in the assessment of spinal metastatic disease, the importance and utility of [18F]FDG-PET cannot be understated.3 Because PET and PET/CT are often used early in the oncologic work-up and for surveillance imaging, it is critical for radiologists to understand malignant and nonmalignant disease patterns and characteristics to make an accurate and useful diagnosis.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received June 22, 2021.
- Accepted after revision August 4, 2021.
- © 2022 by American Journal of Neuroradiology