SUMMARY:
The role of molecular markers is increasingly being recognized for head and neck tumors, ranging from benign lesions like paragangliomas to malignancies like squamous cell carcinomas. Multiple studies have recently validated blood tests for circulating tumor tissue–modified viral human papillomavirus DNA (HPV ct-DNA) for posttreatment surveillance of HPV-driven oropharyngeal squamous cell carcinomas. This technology quantifies fragments of circulating DNA that are shed into the blood stream with very high (>95%) positive and negative predictive values and are also highly sensitive in distinguishing tumor HPV-DNA from a noncancerous source. This study has a cohort of 34 patients with HPV-driven oropharyngeal squamous cell carcinomas, having at least 3 sequential imaging studies and ct-DNA values. The study showed a strong positive correlation between the imaging findings and the ct-DNA level in recurrent HPV-positive oropharyngeal squamous cell carcinomas. Findings also include the 100% negative predictive value of HPV ct-DNA tests to rule out tumor recurrence. At our institution, we are now routinely performing the ct-DNA assay for surveillance of treated HPV oropharyngeal squamous cell carcinomas. Correlation among clinical, radiologic, and biomarker findings are now part of routine discussions during the multidisciplinary tumor boards.
ABBREVIATIONS:
- ct-DNA
- circulating tumor DNA
- HPV
- human papillomavirus
- NCCN
- National Comprehensive Cancer Network
- NPV
- negative predictive value
- PCR
- polymerase chain reaction
- PPV
- positive predictive value
- SCCa
- squamous cell carcinoma(s)
- TTMV
- tumor tissue–modified viral
During the past decade, there has been a gradual shift in the etiology of head and neck squamous cell carcinomas (SCCa), with a decline in cancers secondary to environmental exposures such as tobacco and alcohol and concomitant increase in cancers associated with human papillomavirus (HPV) infection. The incidence of HPV-associated SCCa has risen exponentially during the past decade, especially in high-income countries, now accounting for 71% and 51.8% of all oropharyngeal SCCa in the United States and United Kingdom, respectively.1 Although, the prognosis of this subset of patients is considerably better than that of the non-HPV counterpart, the relapse and recurrence rate is still high, with around 10%–25% of patients presenting with locoregional or distant metastasis in the surveillance period.2,3 The posttreatment surveillance for HPV-driven oropharyngeal SCCa relies on clinical examination, endoscopy, and serial imaging studies as outlined by the National Comprehensive Cancer Network (NCCN) guidelines (Version 3.2024). These include guidelines for short-term (<6 months) and long-term (6 months to 5 years) surveillance. Standard imaging surveillance includes contrast-enhanced CT of the neck and PET/CT 3–6 months posttreatment.4 As per the 2024 NCCN guidelines, the optimal timing for the PET/CT examination is 3–6 months with significant false-positive rates in studies performed before 12 weeks.
Negative PET/CT findings at 3–6 months predict improved overall survival at 2 years. Subsequent follow-up imaging surveillance is, however, variable across institutions with poor consensus.4 Imaging studies are limited in their accuracy in demarcating posttreatment changes versus tumor recurrence, frequently leading to invasive procedures and biopsy for confirmation. Moreover, early and clinically occult recurrent tumor may also be missed on imaging. Circulating tumor DNA (ct-DNA) provides a quantitative measure of the tumor-modified HPV DNA, which is different from noncancerous HPV seen in the general population. Circulating tumor DNA provides an ultrasensitive, quantitative measure of posttreatment tumor status, with studies showing 94%–100% sensitivity and negative predictive values (NPVs) and approximately 95% positive predictive values (PPVs).3,5,6 Tumor-modified HPV ct-DNA (NavDx; Naveris Laboratories) is a unique biomarker of HPV-associated malignancies with quantifiable levels in the blood and saliva of patients. The prevalence of various HPV subtypes differs by geographic region, with HPV 16 being the most common subtype in HPV-driven oropharyngeal SCCa in the United States.1 Routine testing of ct-DNA offers a more complete picture of the tumor status than obtained by imaging studies alone.6,7 In a 3-year longitudinal study by Chera et al,8 100% of posttreated patients (n = 115) with negative ct-DNA had no cancer recurrence, whereas 94% of patients with 2 positive (rising) ct-DNA values had recurrence. A combination of imaging findings and the biomarker assay can provide an exceptionally high level of sensitivity and can complement each other in ambiguous cases.
MATERIALS AND METHODS
After institutional review board approval from the Mayo clinic human subject research office, 38 cases of p16+ (HPV driven) oropharyngeal SCCa were retrospectively identified via a institutional database search, from June 2022 to March 2023. The study included patients with definite p16 positivity, the presence of at least 3 sequential imaging studies, and ct-DNA values, including pretreatment ct-DNA values and imaging. Patients with equivocal p16 status but treated as having HPV-induced SCCa were excluded from the study. The study also excluded patients with no pretreatment ct-DNA values. A total of 34 cases met the inclusion criteria of longitudinal imaging and ct-DNA tests, including baseline, pre- and posttreatment, sequential imaging studies, and ct-DNA values (at least 3). There were 2 patients with equivocal HPV positivity on p16 stains and they were excluded from the study. Two patients with no pretreatment ct-DNA values were also excluded. The Online Supplemental Data provide the details of patients’ age, sex, location of primary tumor, initial stage, and HPV subtype along with treatment details.
Technical Specifications
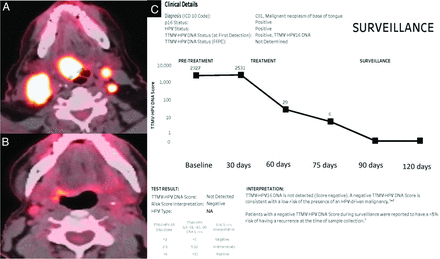
The ct-DNA test isolates circulating free DNA from plasma using droplet digital polymerase chain reaction (PCR) using 17 biomarkers. Millions of single HPV-DNA fragments containing droplets are allocated to 16-size pool clusters, and an algorithm generates a tumor tissue–modified viral (TTMV) HPV DNA prognostic risk score for cancer recurrence. These tests distinguish TTMV HPV DNA from other noncancerous sources of DNA, with results available within 7 days. The test can be used pretreatment, during treatment, and posttreatment. The pretreatment score provides an important baseline score, depending on tumor burden, and identifies the HPV strain. The effectiveness of the treatment response is evaluated by tests during treatment, and finally, posttreatment tests are used for long-term surveillance. The result chart is updated after every test, providing a graph along with absolute values (Fig 1). A TTMV HPV-16 DNA score of <2 is negative, 2–3 is indeterminate, and >3 is positive.
Right base of tongue SCCa (p16 +) with bilateral nodal metastasis with high uptake on initial PET/CT (A) and near-complete treatment response on the follow-up study (B). The ct-DNA results (C) show concordant values with a pretreatment value of 2357 and negative (< 2) results on surveillance with a quantitative value of zero. A mild increase in ct-DNA values is noted on the initial treatment test (2531) secondary to tumor necrosis.
RESULTS
Concordant Negative ct-DNA and Imaging (Most Common) Findings
Twenty-two of 34 patients (64.7%) had negative ct-DNA values obtained during 6–9 months (Fig 1). A clinical examination including endoscopy was performed in all these patients, and findings were negative in all except 1 patient in whom deep ulceration and edema resulted in suboptimal evaluation of the oropharynx.
Discordant ct-DNA and Imaging (Uncommon) Findings
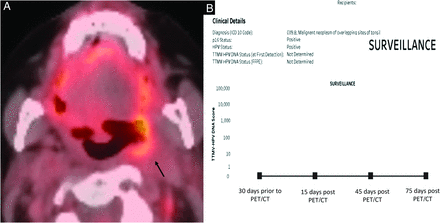
Eight of 34 patients (23.5%) had discordant results. Seven of these 8 patients had negative ct-DNA results and positive or indeterminate findings on imaging, whereas 1 patient had positive ct-DNA findings with negative imaging findings. In the former group (negative ct-DNA and positive imaging), 4 patients had increased oropharyngeal uptake on PET/CT, which was interpreted as “concerning for tumor recurrence” (Fig 2). Two patients had increased nodal uptake, and 1 patient had increased uptake of pulmonary nodules on PET/CT examinations. These (7 of 8) patients with discordant results were followed up with a combination of clinical and endoscopic examinations, ct-DNA, and PET/CT with no findings of recurrence on longitudinal follow-up during the next 4–6 months. A tissue biopsy was deemed unnecessary, given the low suspicion for recurrence based on the negative clinical and endoscopic examination findings and continued negative ct-DNA results. One of 8 had pathology-proved tumor recurrence in which imaging findings were negative, interpreted as “posttreatment changes,” with, however, positive ct-DNA results. The decision to biopsy in this patient was made on the basis of the increasing ct-DNA values.
Discordance between imaging and ct-DNA results. Surveillance PET/CT (A) in a patient with treated p16+ oropharyngeal SCCa shows a nodular focus with increased FDG uptake in the left tonsillar fossa (black arrow), concerning for tumor recurrence. Multiple surveillance ct-DNA tests (B) during the same period, however, showed negative values, and the consensus during the multidisciplinary tumor board was against any intervention. Follow-up PET/CT (not shown) revealed complete resolution of uptake with findings consistent with posttreatment changes.
Concordant Positive ct-DNA and Imaging (Least Common) Findings
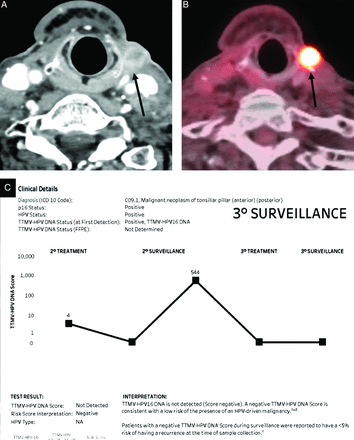
Four of the 24 patients (11.7%) had tumor recurrence detected on PET/CT with positive ct-DNA findings with a temporal increase in values. Two of these recurrences were localized and treated with curative intent (Fig 3), with negative ct-DNA findings on follow-up. The ct-DNA values in these patients with localized recurrent disease were 18 and 56, at 6 months and 9 months, respectively. Two patients had widespread metastases, with progressive disease on imaging and increasing ct-DNA values, treated palliatively. The ct-DNA values in patients with widespread metastasis were 78,445 and 159, with confirmation of recurrence at 9 months.
Recurrence of p16+ oropharyngeal SCCa along the left lateral neck with concordant imaging and ct-DNA results. Surveillance contrast-enhanced neck CT (A) and PET/CT (B) in a patient with treated p16+ oropharyngeal SCCa show nodular enhancement with increased FDG uptake in the left lateral neck (black arrows), concerning for tumor recurrence. A marked increase in values from 0 to 544 was seen on the concurrent ct-DNA test. This increase was treated with surgical resection (curative intent) with a return of ct-DNA values to zero (negative) on follow-up surveillance.
Overall, the negative predictive value and positive predictive value for ct-DNA were 100% (Table), with the latter, however, limited by a small sample size (4 patients with positive values). There were no false-negative ct-DNA cases in this study. There was an increase in the ct-DNA values of 2 patients on the first posttreatment study, presumed to be secondary to high tumor fragmentation, with negative ct-DNA values on subsequent tests.
| Total Cohort (n = 34) 100% | ct-DNA | CT FDG-PET/CT | Long-Term Surveillance or Pathology Outcome | |
|---|---|---|---|---|
| Concordant negative | 22 (64.7%) | 22 (Negative) | 22 (Negative) | Negative on long-term surveillance |
| Discordant | 8 (23.5%) | 7 of 8: Negative ct-DNA results and positive findings on imaging | Negative on long-term surveillance | |
| 1 of 8: Positive ct-DNA results and negative imaging | Positive on pathology | |||
| Concordant positive | 4 (11.7%) | 4 (Positive) | 4 (Positive) | Positive on pathology |
Note:—NPV = 100%, PPV = 100%.
Results with absolute number and percentages of positive and negative imaging findings and CT and/or FDG-PET/CT (total cohort = 34 patients)
DISCUSSION
During the past decade, oropharyngeal SCCa has become the most prevalent cancer associated with HPV in the United States.1 Despite having a much better prognosis compared with non-HPV oropharyngeal SCCa, there is significant risk of recurrence (15%–25%) within the first 5 years of definite therapy.1,2 ct-DNA has emerged as a robust diagnostic tool to detect and quantify HPV-positive tumors.3,5 Conventional ct-DNA could not distinguish HPV DNA, secondary to a wide range of acute or chronic viral infections, from tumor DNA. However, with recent developments, these tests can precisely and specifically measure the cell free TTMV-HPV load using ultrasensitive multianalyte digital droplet PCR assay tests. TTMV HPV DNA is a unique biomarker released in the bloodstream by fragmentation of malignant epithelial cells with HPV-modified DNA.5⇓⇓–8 Studies suggest that the quantity of HPV ct-DNA shed into plasma varies significantly and is dependent on tumor (including nodal) burden, HPV genomic integration, and other tumor features such as proliferation and vascularization.5,9
Around 80%–90% of patients with oropharyngeal SCCa have detectable levels of ct-DNA at time of diagnosis, which is established as a baseline.5 A low baseline level of ct-DNA is indicative of a low tumor HPV copy number and higher rates of HPV genomic integration, both of which are associated with adverse tumor genomic features. In contrast, patients with abundant (>200 copies/mL) ct-DNA levels have a favorable prognosis.5,6 Pretreatment ct-DNA levels correlate strongly with the overall tumor burden, and rapid clearance of the biomarker after chemoradiotherapy predicts the likelihood of disease control.9 Some patients may show a transient increase (“spike”) after initiation of chemoradiation, likely reflecting early tumor cell destruction, and this could have clinical value as a surrogate for early treatment response. The transient elevation in HPV ct-DNA resolves in all patients without recurrent disease.5,10 A serial increase in ct-DNA values provides stronger correlation with tumor recurrence compared with a single time point increase in values. The literature on the role of this marker as a tool for surveillance post-definite therapy is still limited. The NCCN guidelines (Version 3.2024) for surveillance include periodic clinical and imaging examinations in the first 6 months posttreatment (short-term) including CT and/or MR imaging within 3–4 months after surgical treatment for patients with locoregionally advanced disease or with altered anatomy, resulting in a difficult clinical assessment, to establish a new baseline for future comparisons.
FDG-PET/CT is the most sensitive imaging technique and is recommended within 3–6 months of definitive radiation or systemic therapy for assessment of treatment response and to identify any residual tumor. There are no consensus guidelines on the frequency and technique of routine long-term (>6 months to 5 years) posttreatment imaging in the asymptomatic patient, with wide variability across institutions.4 Clinical examinations, including endoscopic evaluation, have limited sensitivity for early detection of tumor recurrence.6 Imaging with contrast-enhanced CT and/or PET forms the mainstay with high sensitivity. PET/CT offers an NPV of almost 100%; however, there is a high false-positive rate (20%–30%) limiting the PPV.11,12 These imaging examinations are limited in specificity secondary to a wide range of factors including scarring, inflammatory changes, and the complex appearance of flaps.
Although the findings of PET and contrast-enhanced neck CT are concordant in most cases, both have limitations when it comes to ambiguous cases.11 Longitudinal assessment of HPV ct-DNA offers a very sensitive noninvasive study, which, when combined with imaging, can offer almost 100% sensitivity and specificity. Sequential positive results during the surveillance phase have exponentially higher sensitivity for recurrence compared with a single positive result.6,7 Although studies are limited at present, the sensitivity, PPV, and NPV of these biomarkers exceed those of any imaging examination. The serologic tests have the potential for early detection of tumor recurrence and can serve as an alternative for invasive tissue biopsies in cases with ambiguous imaging findings.6,7 Moreover, the blood tests can potentially decrease the frequency of radiologic and endoscopic examinations for long-term surveillance of HPV-positive SCCa. Alternatively, upward trends in the serologic marker could prompt an earlier imaging study to localize the site of recurrence.
Patients with oligometastatic disease (1–5 lesions) can be treated with curative intent compared with palliative therapy for widespread metastasis.13 Initial studies, though limited in number, have consistently shown a 94%–100% NPV with a low likelihood of tumor recurrence in the absence of ct-DNA elevation.5 The PPV was also very high (around 90%–95%) in most studies, with some patients developing a transient spike in ct-DNA without recurrence on longitudinal follow-up.3,8 In our study, there were 2 cases of HPV-equivocal SCCa on p16 immunostaining that had undetectable levels of pretreatment ct-DNA, which were excluded. P16 immunohistochemistry is the most widely used test for assessment of HPV causation in oropharyngeal SCCa and serves as an easy and economic surrogate for HPV DNA or RNA testing. However, the tumor may be inaccurately categorized as HPV+ by p16 staining or other HPV assays in up to 20% of cases.14 Discordance between p16 and HPV DNA or RNA status affects patient prognosis in terms of disease-free and overall survival. Recent studies have shown that patients with discordant oropharyngeal SCCa (p16–/HPV DNA or RNA+ or p16+/HPV DNA or RNA–) had a significantly worse prognosis than patients with p16+/HPV DNA or RNA+ oropharyngeal cancer, however, a significantly better prognosis than patients with p16–/HPV DNA or RNA– oropharyngeal SCCa.14
In cases of discordant pretreatment results, repeat testing with a combination of p16 immunohistochemistry and HPV DNA PCR offers high sensitivity for definitive assessment of tumor HPV status. This combination is recommended when the HPV status might influence patient care, especially in geographic regions with low HPV-attributable fractions.5 Detection of HPV oncogene (HPV E6/E7 messenger RNA) transcripts using an amplification-based method constitutes the current criterion standard to identify the etiologic role of HPV in oropharyngeal SCCa. However, considering the comparatively laborious methodology and high cost of these tests, simple HPV DNA PCR with p16 immunohistochemistry serves as a reasonable alternative.15 In the study by Chera et al8 with 115 subjects, 15 patients developed recurrence. In this subset, there were 4 patients in whom imaging failed to detect tumor recurrence because the recurrence was outside the region being imaged in all 4 cases.
The findings in our study are concordant with other published literature. Although limited by a small sample size, this study had a negative predictive value of 100%. It has been <1 year since the tests have been incorporated into routine clinical practice at our center. During the next few years, we expect larger series comparing imaging and serologic tests for surveillance of oropharyngeal SCCa. Discussion of ct-DNA is now an integral part of multidisciplinary tumor boards and offers a great opportunity for detection of early and small-volume tumor recurrence. The economic value of “liquid biopsy” testing is affected by not only the sensitivity and specificity of ct-DNA but also the clinical utility of the study. Clinical utility can be demonstrated by a change in patient outcomes or clinical practice guidelines, such as decreased frequency of physical and imaging examinations and the associated financial burden of outpatient surveillance care.5⇓⇓–8
CONCLUSIONS
Longitudinal monitoring of HPV ct-DNA for posttreatment surveillance of p16+ oropharyngeal SCCa can accurately detect disease recurrence with a potential to decrease the frequency of physical and imaging examinations and modification of the surveillance guidelines. The neuroradiologist should integrate the results of serologic tests into interpretation of surveillance imaging. Additional studies with larger cohorts and a longer time course of follow-up are, however, needed for further validation of these biomarkers.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received January 25, 2024.
- Accepted after revision March 8, 2024.
- © 2024 by American Journal of Neuroradiology