Abstract
BACKGROUND AND PURPOSE: In subjects with subcortical ischemic vascular dementia (SIVD), tissue vacuolization, myelin pallor, and demyelination have been found on pathologic examination of white matter signal hyperintensities (WMSH). Magnetization transfer ratio (MTR) values provide a potential measure of compromised white matter integrity. The purpose of this study was to determine if there were differences in MTR of WMSH between subjects with SIVD and cognitively normal healthy control subjects.
METHODS: Fifteen subjects with SIVD and 16 control subjects of comparable age and sex were studied. MTR images were coregistered to MR images segmented into tissue classes (gray matter, white matter, CSF, WMSH, and lacunar infarcts). MTR of WMSH was compared across groups and examined by WMSH location, size, and total burden.
RESULTS: WMSH burden was greater in SIVD patients than in control subjects (2.4% vs 0.67%). MTR of WMSH did not differ between groups, but MTR of periventricular WMSH was lower in SIVD patients than in control subjects (37.6% vs 39.4%). Even after accounting for covariant effects of lesion burden, there was still a trend toward reduced periventricular WMSH MTR in the group with dementia. There was no correlation between WMSH MTR and WMSH lesion size.
CONCLUSION: These findings are consistent with observations that pathologic changes in vascular dementia are most severe in the periventricular white matter and suggest that insight into the pathophysiology of SIVD might be gleaned from studies of the periventricular region.
Vascular dementia is the second most common cause of dementia in the United States. The patient must meet six criteria for a positive diagnosis (1); among them the presence of cerebrovascular lesions (subcortical, cortical, and lacunar infarcts, and white matter signal hyperintensities [WMSH]) on neuroimaging studies and a temporal relationship between those lesions and intellectual decline (1, 2). Thus, neuroimaging plays a crucial role in the work-up of vascular dementia. WMSH lesions are common in normal aging (3–7), however, and are strongly associated with risk factors for vascular disease (3, 6). Furthermore, there is no simple relationship between WMSH and cognition (8–12), so it is important to identify an independent neuroimaging marker for vascular dementia. WMSH lesions are characterized by variable degrees of gliosis, vacuolization, myelin pallor, and demyelination (5, 13–15). There may be differences in the extent, location, or nature of white matter disease in subjects with vascular dementia as compared with normal aging or Alzheimer dementia (16, 17). T2-weighted MR imaging is sensitive, but not specific, for these various disorders. An MR technique potentially more sensitive (18, 19) and specific (20–22) for detecting white matter demyelination is that of magnetization transfer ratio (MTR) measurements (23).
We tested the hypothesis that MTR of WMSH is lower in subjects with subcortical ischemic vascular dementia (SIVD) than in elderly healthy control subjects. We also investigated the effect of WMSH lesion location, size, and burden on MTR of WMSH. This was accomplished by using a previously described semiautomated technique for examining tissue-specific MTR (24).
Methods
Subjects
Subjects were recruited from a university dementia clinic and from Department of Veterans Affairs hospitals and clinics. Inclusionary criteria were the presence of at least one subcortical lacunar infarct identified on MR images and a clinical dementia rating (CDR) of 1 (0 = cognitively normal, 0.5 = cognitively impaired, 1 = demented, 2 = severely demented). For all subjects, we obtained a general medical history, physical examination, neurologic examination, serum chemistry, blood count, thyroid function tests, and B12 assessment. In addition, comprehensive neuropsychological testing was performed in all subjects to assess general cognitive function, language, visuospatial ability, executive function, memory, and attention. General cognitive function was assessed with the mini-mental status examination (MMSE).
Subjects were excluded if they met any of the following criteria: older than 55 years of age, non-English speaking, severe dementia (CDR > 2), alcohol or substance abuse, severe head trauma, severe medical illness, neurologic or psychiatric disorders, medications that affect cognitive function, abnormal B12 or thyroid metabolism, cortical infarction, or structural brain disease other than lacunar infarcts and WMSH.
The protocol was approved by all institutional review board committees, and all subjects or their legal guardians gave written informed consent before participating in the study.
MR Acquisition
All studies were performed on a 1.5-T MR system using a head coil with quadrature detection. The brain imaging protocol was as follows: 1) proton density—and T2-weighted spin-echo axial images (2500/20,80/1 [TR/TE/excitations]), 3-mm-thick sections with no gap, and 0.94 × 0.94 mm2 in-plane resolution; 2) T1-weighted 3D magnetization prepared rapid gradient-echo coronal images (10/4/1), 1 × 1 mm2 in-plane resolution, and 1.4-mm-thick partitions; and 3) magnetization transfer ratio images with a 3D axial fast gradient-echo sequence that was intermediate between T1- and proton density–weighted (55/6/1), a flip angle of 10°, 4-mm-thick partitions, and 0.94 × 0.94 mm2 in-plane resolution. Although the optimal sequence would be proton density–weighted, a shorter TR was chosen (thus adding some T1 weighting) to reduce scan acquisition time. A 3D data set was obtained without and with magnetization transfer. Saturation was achieved by applying a 7.8-millisecond gaussian-shaped saturation pulse with a magnitude of 9 μT (B1) 1500 Hz downfield of the narrow water resonance. The specific absorption rate was below the FDA limits for all subjects.
Image Analysis
The procedure for image analysis is an extension of previous work performed in this laboratory (24). To summarize, T1- and T2-weighted images were used for segmentation as follows: the skull was stripped from the images, the 3D T1-weighted images were coregistered to the spin-echo dataset (25), a 3D inhomogeneity filter was applied, and the whole brain was segmented into CSF, white matter, and gray matter, using k-means cluster analysis (26). This initial, automated process was followed by manual editing on a section-by-section basis to separate cortical from subcortical gray matter, ventricular from sulcal CSF, and to reclassify pixels incorrectly classified as gray matter as WMSH. Two additional steps in the editing process were then performed. First, lacunar infarcts were manually outlined under the supervision of a neuroradiologist, and second, because it is known that MTR in the compact fibers of the corpus callosum are higher than in other white matter regions, the corpus callosum was excluded from analysis by tracing the smallest rectangular block that enclosed the corpus callosum on the midline sagittal image and extending this block on either side of the midline by 2 cm.
MTR Images
The procedure for calculating MTR images is the same as described in our previous report except that MTR values were computed and analyzed as floating point values (32-bit single precision) to reduce scaling error and rounding error effects.
Combining MTR and Segmentation Data
The MTR datasets were resectiond to match the segmented spin-echo data set and combined with the edited segmentation images. The preceding steps in the analysis are shown graphically in Figure 1A–C.
A–C, A, Proton density–weighted section from a subject with extensive WMSH; B, corresponding segmentation section showing different tissue classes (WMSH in black); C, color-coded map of MTR of WMSH (green corresponds to lowest MTR and yellow to highest MTR, shown in the color bar); and D, a sample of periventricular WMSH (black line, defined as those pixels representing the overlap between WMSH and a two-pixel region grown of ventricular space but excluding all partial volumed pixels (see Methods for details)
Analysis of Periventricular WMSH
WMSH of the periventricular region was analyzed separately from the rest of WMSH in the region of growth within the ventricular space. A within-section two-pixel region growth of the ventricular space produced an image of the ventricle enlarged by approximately 3 mm on each side. The overlap between the WMSH regions and this enlarged ventricular region was defined as periventricular WMSH (Fig 1D). We excluded all pixels of a tissue category that were immediately adjacent to a pixel of another category to reduce partial volume effects for all tissue categories (including periventricular WMSH).
Statistical Analysis
The percentage of tissue classes (normalized to total intracranial vault), MTR of white matter, cortical gray matter, and total and periventricular WMSH were the dependent variables. Tissue contributions were compared using a two-tailed t-test assuming unequal variance. For MTR analysis, median values within subjects were analyzed as a more robust estimate of central tendency. Initial hypothesis testing of MTR data used analysis of variance (ANOVA), and subsequent testing used analysis of covariance (ANCOVA) with the percentage of WMSH and the MTR of periventricular WMSH as covariates. Correlation analyses were performed using Pearson product moment correlations.
Results
Demographics
There was no difference in age or sex between the groups. MMSE scores and years of education were significantly lower in patients with SIVD as compared with control subjects (Table 1).
Demographic data for healthy control subjects and subjects with subcortical ischemic vascular dementia (SIVD)
MTR
MTR of total WMSH was lower than MTR of normal white matter in both SIVD patients and control subjects. MTR of total WMSH did not differ between the groups. On the other hand, MTR of periventricular WMSH was lower in SIVD patients than in control subjects (P < .01) and, in both groups, exhibited a tendency to be lower than MTR of nonperiventricular WMSH (Fig 2; P < .05 for SIVD patients, P < .14 for control subjects) (Table 2). Lower MTR values in the periventricular area can be seen in Figure 1. MTR of cortical gray matter or normal white matter did not differ between SIVD patients and control subjects (Table 2).
Magnetization transfer ratio (MTR) of various tissue classes in healthy control subjects and subjects with subcortical ischemic vascular dementia (SIVD)
MTR of periventricular and nonperiventricular WMSH for control subjects and patients with SIVD. Values are mean ± 1 SD; P values are before correction of the percentage of WMSH
Tissue Segmentation
There was significantly more WMSH (P < .001) and larger ventricular volume (P < .005) in patients with SIVD than in control subjects (Table 3). The remaining tissue categories showed no significant group difference.
Tissue contribution in healthy control subjects and subjects with subcortical ischemic vascular dementia (SIVD)
For MTR of periventricular WMSH (dependent variable), there was no interaction between group and WMSH lesion burden (ie, the correlation between WMSH lesion burden and periventricular WMSH MTR behaved similarly for SIVD and control subjects). ANCOVA showed that, after accounting for covariant effects of lesion burden, there was still a trend toward reduced periventricular WMSH MTR in the group with dementia (P < .08) (Fig 3).
The effect of group and percentage of WMSH on the MTR of periventricular WMSH. Control subjects indicated by filled circles, SIVD patients by open circles.
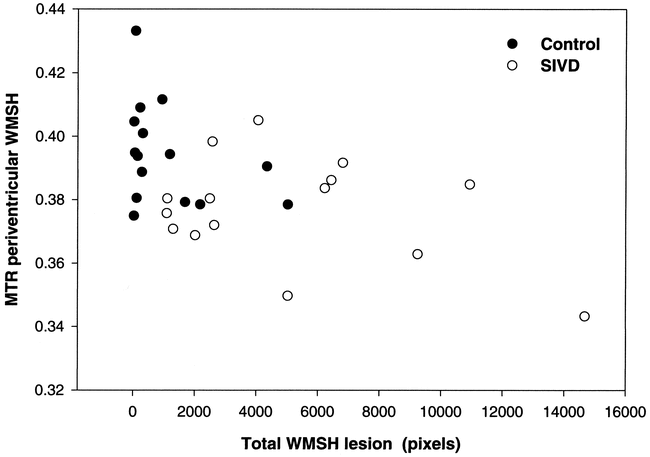
To determine whether the size of individual WMSH lesions affected the MTR, we analyzed individual lesions for each subject and found no correlation between lesion size and corresponding MTR of that lesion (Fig 4). There was also no correlation between MTR of WMSH and ventricular volume and no difference in the histogram distribution of MTR of WMSH.
Plot showing no correlation between lesion size and MTR of that lesion for either control subjects or patients with SIVD. Control subjects indicated by filled circles, SIVD patients by open circles.
We also tested for the possibility that reductions in MTR of periventricular WMSH were an artifact caused by partial volume effects, differences in the abilities of SIVD patients and control subjects to remain motionless during a study, or both. To this end, we examined the MTR of periventricular normal-appearing white matter and found no differences between MTR of normal-appearing periventricular white matter and other normal-appearing nonperiventricular white matter, either in SIVD or control subjects.
There was no correlation between MMSE score and tissue segmentation or MTR measures.
Discussion
Neuroimaging plays a crucial role in the diagnosis of vascular dementia; the imaging findings consist of cortical infarcts, infarcts in strategic locations, or subcortical infarcts (1–2). Subcortical infarcts in white matter are associated with leukoaraiosis (hypodensity on CT scans or WMSH on MR images). It is well known, however, that WMSH are common in older patients without dementia (4–6, 11, 15), especially if they have risk factors for vascular disease (3, 6, 27). Therefore, it is important to identify markers for vascular dementia independent of and more specific than the presence of WMSH alone. MTR is an MR technique potentially sensitive and specific for demyelination, and, since demyelination is an important pathologic feature in SIVD (16, 28), we wanted to determine whether MTR could be useful in the work-up of vascular dementia. We found that, compared with cognitively normal elderly control subjects, subjects with SIVD had a greater WMSH burden, no difference in the MTR of the total WMSH burden, but lower MTR of periventricular WMSH.
Our findings are consistent with the observation that among the white matter regions in the brain, the periventricular area tends to be most affected in vascular dementia (16, 17). Erkinjutti et al (16) found that myelin loss in the periventricular area was more severe in patients with vascular dementia as compared with those with Alzheimer dementia. Ishii et al (17) mapped the location, size, and number of lacunar infarcts in 30 necropsy cases of vascular dementia and found the highest density and frequency of lacunar infarcts in the periventricular frontal lobe and caudate head. Furthermore, studies with positron emission tomography suggest that periventricular WMSH may disrupt connections to the frontal lobe that are important in the development of cognitive decline in vascular dementia (29). The pathologic findings in WMSH in the periventricular region may be distinct from nonperiventricular WMSH. Fazekas et al (15) found areas of demyelination and subependymal gliosis corresponding to periventricular WMSH, while ischemic tissue damage corresponded to confluent deep WMSH. Moody et al (30) were the first to describe periventricular venous collagenosis in brain autopsy specimens from neurologically normal patients. Thus, our results are consistent with the idea that pathologic lesions in the periventricular region are different from that in deep WMSH and, moreover, are accentuated in patients with SIVD.
To determine whether MTR measures are any better than indexes of T1, T2, or proton density, we performed additional analyses comparing T1-, T2-, or proton density–weighted pixel intensities (after scaling each subject's data to the median intensity of ventricular CSF). None of these standard imaging measurements resulted in even a weak trend toward differences in MTR of WMSH or periventricular WMSH between SIVD and control subjects. Thus, MTR appears to be more sensitive than conventional MR measurements.
It is less clear why we did not find reduced MTR for the total volume of WMSH. Significant myelin loss observed in subjects with vascular dementia (16, 28, 31) should be associated with lower MTR measurements. While MTR of periventricular WMSH was lower in SIVD subjects, neither total WMSH nor normal-appearing white matter showed group differences. There are several explanations. First, our findings may reflect more demyelination around the ventricles as compared with deep white matter, indicating that the majority of the WMSH burden does not represent demyelination. Second, the reduced MTR may reflect microscopic lacunar infarcts around the ventricles, too small to be resolved as discrete lacunae but large enough to disrupt saturation effects. Finally, the pathologic structure of WMSH in vascular dementia is incompletely understood and is heterogeneous, at best. The entity of Binswanger disease (subcortical arteriosclerotic encephalopathy) comes to mind (31). Considered a subtype of vascular dementia, Binswanger disease is associated with extensive white matter subcortical infarction (1), but Revesz et al (31) have emphasized that it is not a demyelinating process. Though it is rare, we cannot say for certain whether some of our SIVD patients have Binswanger disease.
Our finding of a greater WMSH burden in SIVD patients as compared with control subjects is consistent with other studies, including findings in more mild forms of cognitive performance (6–8). It is important to bear in mind that no simple relationship between lesion load and dementia exists; thus, there is no consensus on the clinical significance of WMSH.
We found no correlation between WMSH lesion size and the MTR of that lesion (Fig 4). Differences in the histopathologic findings between large (>10 mm) and small white matter lesions has been reported (14). Gliosis and vacuolization characterized large white matter lesions, while dilated perivascular spaces characterized the small lesions. Since perivascular spaces are filled with CSF, we might expect lower MTR in the smaller lesions, but found no evidence of this.
Finally, we investigated whether the distribution of WMSH MTR measurements would be biased toward lower values in SIVD patients; that is, that SIVD would have a heavier “left tail” (24). Others have found a similar analysis to be useful for evaluating MTR of multiple sclerosis lesions (32). We found no difference in the left tail distribution of WMSH MTR in SIVD patients and control subjects.
There are several limitations to this study. The first is the relatively small number of subjects in each group. We used rigorous criteria to diagnose SIVD in an attempt to reduce variability in the populations. As a result, our subjects are well characterized, but small in number. Second, partial volume averaging of different tissue classes could be a problem. Nonetheless, several steps (region growing, shrinking, and pixel exclusion) were taken to reduce partial volume averaging as much as possible. The third limitation is that we do not yet have pathologic confirmation for the diagnosis of SIVD. These data are forthcoming, because the subjects will be followed to autopsy.
Conclusion
Vascular dementia is a heterogeneous clinical entity for which several subtypes exist. We reduced this heterogeneity by studying well-characterized demented subjects with lacunar infarcts and WMSH. We found that MTR of WMSH was not different in subjects with SIVD as compared with the control group, but MTR of periventricular WMSH was lower in the SIVD subjects. Our findings are consistent with observations that, in vascular dementia, pathologic changes (demyelination, vacuolization, and lacunar infarcts) are most severe in the periventricular region. Our results suggest that, while the clinical usefulness of MTR is limited in regard to the diagnosis of SIVD, further insight into the pathophysiology of vascular dementia may be found by neuropathologic investigation of the periventricular region.
Footnotes
↵1 Supported in part by NIH grant P01 AG12535 (H.C.); by the UC Davis Alzheimer's Center AG 10129 (W.J.J.); by the State of California Department of Health Services, Alzheimer Program, National Research Service Award DA-05683-02 (J.T.); by NIH grant R01AG10897 (M.W.W.); and by a Career Scientist Award from the Department of Veterans Affairs (G.F.).
↵2 Address reprint requests to G. Fein, PhD, Psychiatry Research, San Francisco VA Medical Center, 4150 Clement St, San Francisco, CA 94121.
References
- Received October 21, 1998.
- Accepted after revision January 28, 1999.
- Copyright © American Society of Neuroradiology