Abstract
BACKGROUND AND PURPOSE: Fabry disease (FD) is an inborn error of glycosphingolipid metabolism. To date, no specific neuroimaging features have been elucidated to help in making the diagnosis of this disorder. The purpose of this study was to determine whether the finding of T1 shortening in the lateral pulvinar is a useful finding in the imaging diagnosis of FD and to deduce the relationship of this finding to the pathophysiology of the disease.
METHODS: We studied T1- and T2-weighted images obtained in ten patients (nine male and one female) with FD with an age range of 19–59 years. The images were examined for anatomic aberrations and areas of abnormal signal intensity (SI) in both gray matter and white matter. The SI of deep gray matter was evaluated qualitatively and semiquantitatively, relative to the SI of CSF or the genu of the corpus callosum. Gradient echo MR images and axial noncontrast CT images were available for one patient.
RESULTS: Seven of 10 patients showed small areas of T2 prolongation in the white matter of the cerebral hemispheres. Despite the known propensity for vascular disease in these patients, only one had cortical infarction. Bilateral T1 shortening in the lateral pulvinar was recognized in at least seven patients, all over the age of 30 years, who also had small areas of T2 prolongation in the white matter. CT and gradient echo images in one patient revealed no evidence of calcification or metallic deposits in the pulvinar.
CONCLUSION: Bilateral T1 shortening in the lateral pulvinar is a common finding in FD and may be useful in suggesting this diagnosis.
Fabry disease (FD) is an X-linked disorder of glycosphingolipid metabolism (1). Deficient activity of the lysosomal hydrolase, α-galactosidase A, leads to progressive accumulation of glycosphingolipids, predominantly ceramide trihexoside, in the vascular endothelium, perithelium, and smooth muscle cells, as well as in parenchymal cells in the kidney, heart, dorsal root ganglia, autonomic nervous system, and brain (2–6). Progressive endothelial glycosphingolipid accumulation results in compromise of lumen size and subsequent vascular events such as myocardial ischemia and stroke. These events typically occur in the late fourth through early sixth decades of life (1).
The effects of the cerebral vasculopathy of FD are well visualized on MR images. T2-weighted images show an increasing number of deep white matter and deep gray matter high signal intensity (SI) lesions that may coalesce over time (7, 8). Autopsies have revealed that these deep white matter lesions are lacunar infarctions or are associated with narrowing of small arterioles (1–3, 9). With age, larger vessels are affected, and infarctions involving the cortical gray matter also appear (7). The tendency for larger cortical lesions to occur in the vertebrobasilar territory could be explained by lumen compromise superimposed on the ectasia found in these vessels (7, 10). Using Kaplan-Meier estimates, Crutchfield et al (7) found that in a group of FD patients the mean age when a lesion of variable location was disclosed by MR imaging was 43 years (38 years for 25% prevalence, 43 year for 50% prevalence, and 48 years for 75% prevalence), which is slightly earlier than the development of T2 hyperintensities in normal populations. However, small T2 hyperintensities of the periventricular white matter and deep gray matter are common, nonspecific findings and, as such, are not useful in establishing, or even suggesting, a diagnosis of FD. Furthermore, only 37.5% of the patients with lesions visualized on MR images were symptomatic. We herein report our study of brain MR imaging in a cohort of patients with FD. We suggest that specific MR imaging findings may allow an early diagnosis of FD and perhaps allow early intervention to help to prevent or delay the later complications of the disease.
Methods
Inclusion Criteria
We studied 10 patients (nine male, one female) with FD, 19–59 years of age (Table 1). The diagnosis of FD was confirmed by assay of leukocyte α-galactosidase A enzyme activity and, in some instances, mutation identification. Central nervous system symptoms of these patients are described in Table 1. Most of the symptoms were transient, suggesting transient ischemic attack, except for double vision in patient 5 and aphasia in patient 10. No participant had hepatic dysfunction. The study protocol was approved by our institutional review board, and all subjects gave written informed consent for their participation.
Clinical and MR imaging findings in Fabry disease
Imaging Techniques
All MR imaging studies were performed with a 1.5-T magnet. Axial spin-echo T1-weighted images (500/12/2 [TR/TE/NEX]) and conventional spin-echo T2-weighted images (2500/80/1) were obtained. Conventional spin-echo sequences have the advantage of better contrast-to-noise ratio, which is important in evaluating subtle cortical and white matter abnormalities. In a single patient (patient 10), gradient-echo images (500/25; θ = 30), diffusion-weighted echo planar imaging (7000/100; b = 800), and brain CT (contiguous axial 3.75-mm images without contrast medium administration) were also performed.
Image Interpretation
All images were reviewed, individually and jointly, by two reviewers (J.T., A.J.B.) with experience in neuroimaging. When disagreements occurred, they were resolved by consensus. In particular, the images were reviewed for the presence of T2 high-SI abnormalities in the cerebral (periventricular, deep, subcortical) and cerebellar white matter or deep gray matter; evidence of ischemic infarction (cortical atrophy or T1 shortening with T2 prolongation in the underlying white matter); and SI abnormalities in the deep gray matter (globus pallidus, putamen, thalamus, substantia nigra, red nucleus, and cerebellar dentate nucleus). T1 and T2 shortening were considered qualitatively abnormal when a structure was more hyperintense or hypointense on T1- or T2-weighted images than the SI expected for age, as compared with normal standards (11). We also semiquantitatively analyzed ratios of SI of the deep gray matter structures relative to those of cerebrospinal fluid in the lateral ventricle or genu of the corpus callosum (CC). To perform this assessment, circular regions of interest were placed over each deep nuclear structure, as well as over the frontal horn of the lateral ventricle and the callosal genu. The SI from each measurement was recorded, and then SI ratios were calculated for each deep nuclear structure by using both the frontal horn and the callosal genu as the denominator. For age-matched controls, we reviewed MR studies of 40 adult patients, 20–60 years of age (10 patients, 20–30 years of age; 10, 30–40 years of age; 10, 40–50 years of age; and 10, 50–60 years of age). These control patients were imaged because of headaches, seizures, or neck problems; all had normal MR imaging findings, normal neurologic examination results, and a history of normal development. SI ratios were considered abnormal if they differed by more than 2 SD from the SI ratios of age-matched controls.
Results
The clinical data and MR findings in each FD patient are presented in Tables 1 and 2. Seven of 10 patients showed small foci of T2 prolongation in the white matter. In patient 9, the abnormalities were so subtle that they were judged to be normal for age. In patient 10, who had had a clinical stroke, MR imaging also revealed extensive chronic infarctions (without reduced diffusion) in the left parietal and both frontal and occipital lobes. No acute infarctions were identified.
Signal intensity of deep gray matter
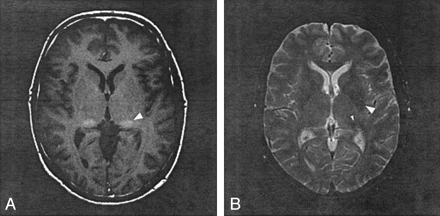
Nearly identical oval-shaped regions of prominent T1 shortening were qualitatively recognized in the lateral aspect of the posterior thalamus (the lateral pulvinar) bilaterally in eight patients with FD (Figs 1A and 2A). Abnormal T1 shortening was recognized in the pulvinar in seven patients by means of SI ratio analysis (relative to both CSF and CC). T2 shortening was identified in the pulvinar in three patients by qualitative analysis, in six patients by SI ratio analysis relative to CSF, and in two patients by SI ratio analysis relative to CC (Table 2). CT and gradient echo images, which were available in patient 10, showed no SI abnormalities in the pulvinar (Fig 1C–E).
Patient 10.
A, Axial T1-weighted spin-echo image at the level of basal ganglia shows hyperintense lesions in the bilateral peripheral globus pallidus, lateral pulvinar (arrowhead), and medial occipital cortex.
B, Axial T2-weighted image spin-echo showing hypointensity in the bilateral globus pallidus (small arrowhead) and putamen (arrowhead).
C, Noncontrast axial CT scan reveals calcification in the bilateral globus pallidus and medial occipital cortex; however, no abnormal attenuation exists in the pulvinar.
D and E, Coronal gradient echo images show hypointensity in the putamen and substantia nigra (D, arrowhead) but normal SI in pulvinar (E).
Patient 8.
A, T1-weighted spin-echo image at the level of basal ganglia shows hyperintensity in right globus pallidus and bilateral lateral pulvinar (arrowhead).
B, T2-weighted spin-echo image shows a small hyperintense foci in right frontal white matter and hypointensity in the bilateral lateral putamen (arrowhead) and pulvinar (small arrowhead).
Qualitative analysis revealed peripheral T1 shortening in the globus pallidus in five patients; in three of them, central T2 shortening of the globi palladi was also identified. Abnormal T1 and T2 shortening in the globus pallidus were detected in three (T1 shortening) and nine patients (T2 shortening) by use of SI ratio analysis relative to CSF and in one (T1 shortening) and six patients (T2 shortening) by use of SI ratio analysis relative to CC (Figs 1A and B, 2A and B). In the putamen, abnormal T1 and T2 shortening were detected in zero (T1 shortening) and three patients (T2 shortening) by use of qualitative analysis, in two (T1 shortening) and seven patients (T2 shortening) by use of SI ratio analysis relative to CSF, and in zero (T1 shortening) and one patient (T2 shortening) by use of SI ratio analysis relative to the CC (Figs 1A and B, 2A and B). Analyses of the other deep gray nuclei by using both qualitative and semiquantitative methods are described in Table 2. In patient 10, CT showed hyperattenuation in the bilateral globus pallidus and medial occipital cortex (Fig 1C), and gradient echo images showed hypointensity in the globi palladi, which suggests the presence of senescent calcification. No SI abnormality was identified in the putamen, substantia nigra, or dentate nucleus on CT scans, whereas hypointensity was present in those regions on the gradient echo MR images (Fig 1D).
Discussion
This study suggests that abnormal SI in the deep gray nuclei is common in patients with FD and that the presence of T1 shortening in the lateral pulvinar is a common and sensitive finding in patients with this disease. Our review of the literature pertaining to FD revealed no previous mention of thalamic involvement. T1 shortening in the pulvinar was recognized in all seven patients over the age of 30 years. This finding was substantiated by use of three qualitative and semiquantitative methods to evaluate T1 and T2 abnormalities in the deep cerebral and cerebellar nuclei. Other neurologic disorders have been described to manifest T1 shortening in the thalamus, including profound asphyxia (12), postanoxic Parkinsonism (13), acute necrotizing encephalopathy (14), Tay-Sachs disease (15), and Krabbe disease (16). In the usual clinical circumstances, however, these other disorders are easily distinguished from FD by clinical consideration and MR imaging findings in brain regions other than the thalamus. Symmetric T2 prolongation in the pulvinar is characteristic of both sporadic and variant Creutzfeldt-Jacob disease, in which setting it is known as the “pulvinar sign;” however, the T1-weighted imaging findings are usually normal (17, 18).
Histochemical studies from brain tissue of patients with FD have revealed an accumulation of glycosphingolipids, predominantly ceramide trihexoside, within selected neurons in the spinal cord and ganglia, brain stem, amygdala, hypothalamus, and layers 5 and 6 of cerebral cortex (2). These regions, however, showed no SI abnormality on MR images. On the other hand, histochemistry reportedly shows no abnormalities in the striatum, globus pallidus, or thalamus (2), regions that show SI changes on the MR studies of our patients. Thus, it seems unlikely that accumulation of ceramide trihexoside, per se, accounts for the T1 and T2 changes that we observed.
One possible cause of T1 and T2 shortening on MR images is calcification. The presence of certain calcium salts in the brain causes alterations in water proton relaxation times that are believed to be related to adsorption of water by the crystalline structure of the calcium salts (surface effect; 19). Calcification is commonly seen in the globus pallidus as part of the aging process (20, 21). Calcification of deep nuclei other than the pallidum has been described in metabolic and endocrine disorders (21). At least three (patients 4, 6, 9) of our seven patients with T1 shortening in the pulvinar had no abnormal SI suggesting calcification in the globus pallidus, which indicates that the pulvinar SI was not senescent calcification. In addition, the CT and gradient echo MR images of patient 10, in whom T1 shortening was observed in both globus pallidus and pulvinar, showed evidence of calcification only in the former (Fig 1C). Moreover, the pattern of T1 shortening in the pallidum was different from that in the pulvinar, the former being patchy around a central hypointensity, whereas the latter was homogeneous in all patients. Because patient 10 was 59 years old at the time of the study, the pallidal calcification could be senescent, but the SI homogeneity and absence of CT evidence of calcium in the pulvinar suggest that, unless MR is more sensitive than CT to low concentrations of calcium salts, the T1 shortening in the pulvinar has a cause other than calcium and a different pathogenesis.
What are other potential causes of the T1 shortening in the pulvinar of our FD patients? T1 shortening can result from interaction of water molecules with large surrounding molecules (as in proteinaceous fluid), causing slowing of water motion, or from direct interactions of water protons with unpaired outer-shell electrons of ions, allowing an electron-proton spin-spin interaction (as with gadolinium enhancement). In clinical MR, it is most commonly attributed to interaction of water protons with products of hemorrhage or trace metals, such as manganese or copper, or free radicals such as melanin (22, 23). Lipid protons have short T1 relaxation times and are seen as hyperintense on MR images, although they have a different chemical shift than water molecules. Thus, lipids can usually be identified by an associated chemical shift artifact. Alternatively, a fat-suppression pulse can be used to eliminate SI and definitively identify an imaging finding as a lipid. Hyperintense findings may also result from locally high cellularity as well as hamartomas and infarcted cortex (so-called laminar necrosis) for unknown reasons (12, 24). In our patients with FD, petechial hemorrhage or hemorrhagic infarction seems unlikely because of the normal CT and gradient echo images in patient 10, whose T1-weighted images were identical to those of the other patients in the series. We were unable to definitively eliminate lipid as a cause, because fat-suppression pulses were not used in any of the patients; however, in view of the absence of chemical shift artifact and the absence of any fat on the CT scan of patient 10, it seems that lipid is an unlikely cause of the SI abnormality. Thus, hypercellularity, ionic interactions, or interactions with free radicals are possible causes that need to be considered. Proton MR spectroscopy in patients with FD shows a widespread reduction of N-acetylaspartate, but no specific finding in the thalami (25). In addition, there has been no known neuropathologic abnormality described in the pulvinar that might cause the T1 shortening described above (2–6). Further neuropathologic evaluation, as well as neuroradiologic assessment, including fat-suppressed sequences and, perhaps, molecular biologic assessment will be necessary to clarify the pathophysiology of the T1 shortening.
Abnormal T2 shortening in the putamen and other deep gray matter structures was observed in some patients by both qualitative and semiquantitative methods, although the incidence of the abnormality was not as consistent among the three methods as was the T1 shortening in the pulvinar. Prominent T2 shortening of the putamen has been reported in patients with various degenerative disorders of the central nervous system as the result of iron accumulation, possibly due to increased axonal iron transport (26). The CT (normal) and gradient echo imaging (marked hypointensity) findings in the putamen, substantia nigra, and dentate nuclei in patient 10 were compatible with iron deposition. T1 shortening, however, was also recognized in the substantia nigra, red nuclei, and dentate nuclei in some patients with FD. Because the T1 shortening effect of iron is much weaker than the T2 effect (26), the T1 shortening in these regions cannot be explained solely by iron deposition. We hypothesize that additional factors, possibly the same mechanisms as those at work in the pulvinar, may play a role in the T1 shortening of these regions.
We elected to use both semiquantitative and qualitative (visual inspection) methods in this study because subtle changes in SI may be difficult to perceive in individual cases. In addition, because the SI of the deep nuclei change in relation to that of the white matter during development and aging (11), we compared our patients with age-matched controls. We believe that, by using semiquantitative methods and requiring SI ratios more than 2 SD from the mean, we have probably decreased the sensitivity of detecting abnormalities of the deep nuclei. Indeed, we thought that patient 2 had abnormal T1 shortening of the pulvinar, but the SI differed from the norm by less than 2 SD by using semiquantitative methods. Had we classified findings in this patient as abnormal, our results would have indicated that 80% of our patients had abnormal pulvinar SI. In addition, it is possible that our semiquantitative method could give spurious results. The SI ratio relative to CSF may be influenced by the CSF motion or partial volume effect, neither of which is necessarily consistent among patients. The SI ratio relative to the CC as the denominator may vary with age or may not be completely normal in FD patients, even though no abnormal SI lesion could be recognized on routine MR images. As a result of the potential problems with both quantitative methods and the qualitative method, the data were analyzed by using all three (one qualitative and two semiquantitative) methods. The consistency of the findings among the three methods as compared with a group of age-matched controls strongly suggests that the observations are valid.
The pulvinar is generally thought to have little important function in humans. Recent articles have suggested a role in spatial coding of vision (27). It may be of interest to test spatial coding in patients with FD and pulvinar T1 shortening on MR images to determine whether these areas of SI abnormality impair function of the affected regions.
Recent data have demonstrated that enzyme-replacement therapy with α-galactosidase A may represent a major clinical advance in the treatment of patients with FD (28–30). In two sets of clinical trials, patients variously showed that enzyme-replacement therapy reduced neuropathic pain, increased creatinine clearance, decreased microvascular endothelial deposits of globotriaosylceramide in the kidney, skin, and heart, and reduced the QRS interval during electrocardiography. A positron emission tomography study also revealed normalization of abnormal cerebral perfusion and vascular reactivity (31); however, the diagnosis of FD is often delayed until renal failure or the cerebrovascular complications of the disease develop (32). For example, FD was misdiagnosed in one patient as multiple sclerosis because of a waxing and waning clinical course and neuroradiologic findings of patchy T2 prolongation in the white matter (9). To help prevent the more severe complications of the disease, and because more than 60% of FD patients with brain lesions shown on MR images have had no symptomatic stroke (7), we stress that it is essential to suspect FD when bilateral T1 shortening in the pulvinar is recognized at MR imaging.
Conclusion
We evaluated MR imaging studies of 10 patients with FD to describe neuroradiologic findings of this disorder, to determine whether any neuroradiologic findings help the physician to suggest this diagnosis, and to deduce the relationship of the findings to the pathophysiology of the brain involvement in FD. Seven of 10 patients showed small areas of T2 prolongation in the white matter of the cerebral hemispheres. Despite the known propensity for vascular disease in these patients, only one had cortical infarction. Bilateral T1 shortening in the lateral pulvinar was recognized in at least seven patients, all over the age of 30 years, who also had small areas of T2 prolongation in the white matter. CT and gradient echo images obtained in one patient revealed no evidence of calcification or metallic deposits in the pulvinar. Bilateral T1 shortening in the lateral pulvinar is a common finding in FD and may be useful in suggesting this diagnosis.
Acknowledgments
We wish to thank the patients and their families for their contribution to this study. We also thank Dr. Masaharu Hayashi (Department of Clinical Neuropathology, Tokyo Metropolitan Institute for Neuroscience, Tokyo, Japan) for helpful advice. This work was supported in part by funding from the Niemann-Pick Foundation, the California Genetic Disease Branch, the Genzyme Corporation, and by National Institutes of Health (NIH) grant M01RR01271 to the University of California, San Francisco, Pediatric Clinical Research Center. E.H.S. is supported by a Neurological Sciences Academic Development Award from the NIH.
References
- Received August 22, 2002.
- Accepted after revision December 16, 2002.
- Copyright © American Society of Neuroradiology