Abstract
BACKGROUND AND PURPOSE: Low birth weight preterm infants are at high risk of brain injury, particularly injury to the white matter. Diffusion tensor imaging is thought to be more sensitive than conventional MR imaging for detecting subtle white matter abnormalities. The objective of this study was to examine whether diffusion tensor imaging could detect abnormalities that may be associated with later neurologic abnormalities in infants with otherwise normal or minimally abnormal conventional MR imaging findings.
METHODS: We prospectively studied 137 low birth weight (<1800 g) preterm infants. Neonatal conventional MR imaging and diffusion tensor imaging were performed near term-equivalent age before discharge, and neurologic development of the infants was later followed up at 18 to 24 months of age.
RESULTS: Among the preterm infants who were fully studied, 63 underwent normal conventional MR imaging. Three of these infants developed cerebral palsy, and 10 others showed abnormal neurologic outcome. Diffusion tensor imaging results for these infants showed a significant reduction of fractional anisotropy in the posterior limb of the internal capsule in neurologically abnormal infants (including those with cerebral palsy) compared with control preterm infants with normal neurologic outcomes.
CONCLUSION: These results suggest that neonatal diffusion tensor imaging may allow earlier detection of specific anatomic findings of microstructural abnormalities in infants at risk for neurologic abnormalities and disability. The combination of conventional MR imaging and diffusion tensor imaging may increase the predictive value of neonatal MR imaging for later neurologic outcome abnormalities and may become the basis for future interventional clinical studies to improve outcomes.
Preterm infants are at high risk for permanent neurologic insults, which may involve visual, auditory, cognitive, behavioral, and motor abnormalities, including cerebral palsy (CP). Macrostructural brain abnormalities often are visible on conventional MR images at term and include focal abnormalities, such as parenchymal infarction, porencephalic cysts, ventricular wall irregularities, and subependymal hemorrhage (1, 2). Diffuse brain abnormalities (such as ventriculomegaly), diffuse white matter atrophy injury (such as periventricular leukomalacia), and prominent Virchow-Robin spaces may also be seen (3, 4). Macrostructural abnormalities visible on images obtained at term have been shown to correlate with the type and severity of neurologic sequelae (5, 6). However, conventional MR imaging lacks sensitivity for detecting injury during the neonatal period; ≤18% of preterm infants who develop CP have no parenchymal abnormalities revealed by early imaging (1). This is, in part, because of the relatively high water content of the immature, unmyelinated white matter, resulting in long T1 and T2 TR, similar to that associated with injury.
Diffusion tensor imaging is a promising new MR imaging technique for the assessment of brain structural integrity and connectivity (7). Based on the diffusion tensor, several quantifiable and absolute measures can be determined and mapped, including fractional anisotropy (FA) and apparent diffusion coefficient (ADC), which are sensitive to microstructural abnormalities that are occult on conventional MR images. Diffusion tensor imaging of otherwise normal appearing white matter has proved useful, largely in adults, in cases of ischemic disease (8), demyelinating disorders (9), multiple sclerosis (10), and normal aging (11, 12). Diffusion tensor imaging has also been used for quantitative measurements of white matter anisotropy in preterm and term infants (13–15). In this age group, anisotropic properties of white matter tracts are thought to relate to premyelination of oligodendroglial and axonal bundle changes (14, 16).
For this study, we decided to focus on a population of preterm infants with minimal or no parenchymal abnormalities revealed by conventional MR imaging obtained near term gestational age. The purpose of this study was to determine whether diffusion tensor imaging can predict which of these preterm infants will later develop CP or have abnormal results of neurologic assessment by measuring global and regional differences in white matter microstructure.
Methods
Patient Population
At Lucile Packard Children’s Hospital at Stanford, MR imaging is routinely performed for the clinical assessment of the brain status of preterm infants before discharge. The pre-discharge MR imaging examination is intended as a clinical study that may be helpful in the management of the infants. Informed written consent was obtained from the parents, or guardians, of all patients to obtain permission for the analysis of the imaging data and clinical follow-up information for research purposes. The clinical MR imaging was performed without sedation, and additional diffusion tensor imaging sequences were obtained as part of the clinical protocol. The study was approved by the Stanford University Administrative Panel on Human Subjects in Medical Research. Patients underwent imaging immediately after a feeding. Ear plugs (MiniMuffs, Natus) to reduce the noise by 50% were applied, and the patients were bundled to preserve warmth, maintain sleep, and reduce patient motion. Our study population was selected from the 137 patients who met our inclusion criteria: gestational age, ≤33 weeks; birth weight, <1800 g; no congenital brain malformation; images obtained between 34 and 42 weeks of age from June 1998 to December 2000. Thirty patients were excluded because of incomplete follow-up data. Of the 107 remaining patients, 17 were excluded because of gross macrostructural abnormalities shown by conventional MR imaging. An additional 27 were excluded because the diffusion tensor imaging acquisitions were incomplete or degraded by patient motion artifact. The final cohort for diffusion tensor imaging analysis was comprised of 63 patients (Table 1).
Study population MR imaging and neurologic follow-up
MR Imaging Acquisition and Analysis
All studies were obtained by using a 1.5-T magnet (General Electric Medical Systems, Milwaukee, WI). All examinations included 4-mm axial sections of conventional fast spin-echo T1-weighted (500/20 [TR/TE]) and T2-weighted (4000/102) sequences and fluid-attenuated inversion recovery (9000/100; inversion time, 2200 ms), gradient-echo (500/15; flip angle, 15 degrees), and sagittal view sequences. Imaging was performed at or near term age (range, 34.2–42.2 weeks; mean, 37 weeks). The conventional MR imaging examinations were interpreted by an experienced pediatric neuroradiologist (P.D.B.) who categorized the findings as follows: C1 = no abnormality; C2 = minimal subependymal hemorrhage or mineralization with no or mild ventriculomegaly; C3 = moderate to severe ventriculomegaly; C4 = parenchymal abnormality, including evidence of injury due to hemorrhage or ischemia (eg, periventricular leukomalacia).
Diffusion tensor imaging was performed by using a single shot spin-echo diffusion-weighted echo-planar imaging technique (16 contiguous axial view 4-mm sections; 7000/106; field of view acquisition matrix, 128 × 128). Diffusion was measured in six noncollinear directions: (x,y,z) = (1,1,0), (0,1,1), (1,0,1), (−1,1,0), (0,−1,1), and (1,0,−1). Four images were acquired and averaged for each gradient direction, with a b value of 1000 s/mm2. Two images without diffusion weighting (B0) were acquired with b = 0 s/mm2. Images were processed off-line on a Sun workstation by using in-house software (Tensorcalc). This program is used to check for motion-corrupted images, to unwarp eddy current distortions by using the B0 and fluid-attenuated inversion recovery images (17), and to calculate diffusion tensor imaging parameters on a voxel-by-voxel basis. ADC and FA values were calculated after diagonalization of the diffusion tensor matrix based on the Stejskal-Tanner equation (18). Whole brain histograms and individual region-of-interest ADC and FA measurements were obtained.
Whole Brain Histogram
After semi-manual stripping of the posterior fossa and cranial structures, whole brain ADC and FA normalized histograms were generated for the cerebrum. All images in the sequence were stripped of the calvarium, using high signal intensity subarachnoid CSF as a boundary marker on B0 images, with the use of MR-Vision software. Further manipulation was required to remove cerebellar and brain stem parenchyma from the most caudal images.
Regions of Interest Analysis
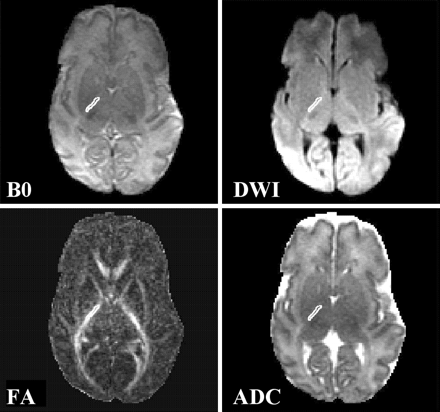
FA and ADC measurements were obtained for the following regions of interest: supraventricular cerebral white matter, genu of the corpus callosum, splenium of the corpus callosum, and anterior and posterior limbs of the internal capsules, caudate, lenticular nucleus, and thalamus. Because image unwarping was accomplished by using B0 images, region-of-interest boundaries were drawn on the B0 images while displayed concurrently on the average diffusion images, ADC maps, and FA maps. The use of B0 images (rather than fluid-attenuated inversion recovery or fast spin-echo images) for image co-registration also eliminates region-of-interest discrepancies due to patient motion between acquisitions. The region-of-interest boundaries were drawn manually and were completely contained within the structure of interest on all sequences (Fig 1).
Images show region of interest placement for the posterior limb of the internal capsule. B0, image without diffusion weighting; DWI, diffusion-weighted image; FA, fractional anisotropy image; ADC, apparent diffusion coefficient map.
Neurologic Outcome
Our clinical end points were based on findings of a detailed neurologic examination performed between 18 and 24 months of corrected age (mean, 20.0 months) by a certified developmental specialist (National Institute of Child Health and Human Development Neonatal Research Network) (19). Using the Amiel-Tison standardized neurologic examination, CP neurologic abnormalities were characterized by abnormal muscle tone/movement/reflex in at least one extremity and abnormal control of movement and posture/gait. CP was diagnosed based on presence of spasticity in at least one extremity. Gross motor skill assessment was based on information presented by Russell et al (20) and Palisano et al (21). Fifty infants had completely normal results of age-appropriate neurologic examination. Three patients met the clinical criteria for CP. Ten infants who had abnormal results of neurologic evaluation but did not meet age or clinical criteria for CP were categorized as infants with abnormal neurologic outcomes. This latter group likely represents a heterogeneous patient population, including patients who may go on to develop CP and patients who may show residual neurologic deficits without clinical criteria for CP. The presence or absence of cognitive delay, speech delay, or subtle fine motor skill deficits was not considered in the present study. Patient outcomes based on conventional MR imaging findings are summarized in Table 2.
Neurologic outcome and conventional MR imaging category for 63 infants fully studied
Statistical Analysis
Diffusion tensor imaging analysis was limited to the 63 patients with minimal or no macrostructural abnormalities revealed by conventional MR imaging (ie, categories 1 or 2) and with usable diffusion tensor imaging sequences. For histogram analysis, comparisons were made for global average FA and ADC means, peak heights, peak locations, and histogram quartiles by using unpaired Student’s t tests. For region-of-interest analysis, an unpaired Student’s t test was used to compare mean FA and ADC values within each region of interest among the clinical subgroups.
Results
Neurologic Outcome
Fifty infants had completely normal results of age-appropriate neurologic examination. Thirteen infants developed neurologic abnormalities. The abnormalities included hypertonia/hyperreflexia, abnormal gait, including toe walking and ataxia, fisting, and asymmetry in the extremities, with three cases of CP having spastic hemiplegia or diplegia. The MR imaging measurements of children with neurologic abnormalities were pooled and evaluated for statistical differences from data obtained from preterm infants with normal neurologic outcomes. The presence or absence of cognitive delay, speech delay, or subtle fine motor skill deficits was not considered in the present study. Patient outcomes based on conventional MR imaging findings are summarized in Table 2.
Histogram Analysis
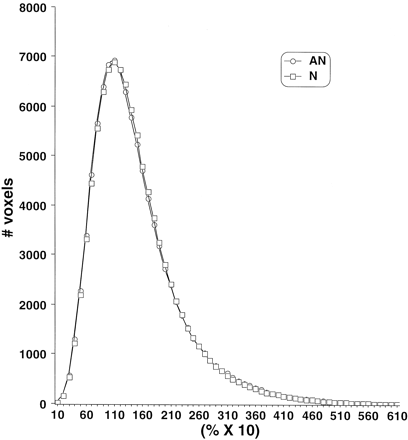
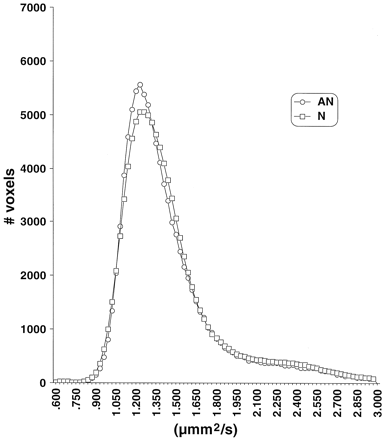
Analysis of the FA and ADC histograms showed no consistent pattern correlating with clinical outcomes (Figs 2 and 3). Comparisons of FA and ADC means, peak locations, and peak values failed to show any differences among the abnormal and normal clinical subgroups. Analysis of the ADC histograms showed a tendency toward lower ADC values among patients with CP compared with normal control patients, but this did not achieve statistical significance (Fig 3).
Average FA histograms by clinical subgroup. N, normal neurologic outcome; AN abnormal neurologic outcome.
Average ADC histograms by clinical subgroup. N, normal neurologic outcome; AN abnormal neurologic outcome.
Regional Analysis
The mean, SD, and range of FA and ADC values of different regions of interest are shown in Tables 3 and 4. FA values for the right posterior limb of the internal capsule were lower in patients with CP compared with normal control patients (P = .0006). Similarly, patients with abnormal neurologic outcomes had lower FA values for the right posterior limb of the internal capsule compared with normal control patients (P = .005). In the left posterior limb of the internal capsule, a statistically significant reduction was noted in the mean FA values comparing the abnormal outcome group with the normal outcome group (P = .03), with similar trends noted in the anterior limb (Table 3). In the right anterior limb of the internal capsule, increased FA values were found in patients with CP compared with control patients (P = .01). Furthermore, FA values in the left cerebral white matter were significantly lower in the abnormal outcome group versus the normal outcome group (P = .049); a trend was also found for the right cerebral white matter (Table 3). ADC analysis showed a trend toward higher values in both right and left cerebral white matter in the abnormal versus normal outcome group. No statistically significant differences among the clinical subgroups for any other region of interest were found (Table 4).
Region of interest fractional anisotropy values by pooled clinical subgroup
Region of interest apparent diffusion coefficient (μ mm2/s) values by pooled clinical subgroup
Because the number of infants with CP was small and a larger number of infants had abnormal neurologic outcomes but not CP and because the purpose of the study was to relate diffusion tensor imaging findings with any neurologic abnormalities, additional statistical analysis was conducted by combining the abnormal outcome and CP patient groups into a single group. Unpaired Student’s t tests comparing the combined group with the normal outcome group showed a significant reduction in the mean FA value in the right posterior limb of the internal capsule (P = .0002). A trend was also found for the left posterior limb of the internal capsule (Table 3). ADC analysis revealed no statistically significant differences among the clinical subgroups for any region of interest except for a trend for higher values in the splenium of corpus callosum in the combined group (Table 4). No significant differences in ADC or FA values were found in caudate, lenticular nucleus, or thalamus among the clinical subgroups (data not shown).
Discussion
Conventional MR Imaging Findings
Conventional MR imaging findings for preterm infants have been shown to correlate with the type and severity of neurologic outcomes (1, 5, 6). Fast spin-echo, T1- and T2-weighted fast spin-echo, fluid-attenuated inversion recovery, and gradient-echo images were acquired for all our patients. For the purposes of this study, the presence of subependymal low signal intensity abnormality was considered to be representative of previous intraventricular hemorrhage rather than a focal parenchymal finding. This convention is supported by the findings of other authors who have further shown that isolated subependymal signal intensity abnormality does not correlate with the presence, type, or extent of neurologic deficit (1). Subtle areas of subependymal signal intensity abnormality suggestive of hemorrhage or mineralization may be imperceptible on conventional T1- and T2-weighted images, although they are visible on gradient-echo and echo-planar images.
It is important to emphasize that in our prospective study, 3 patients with CP and 10 infants with abnormal neurologic findings were among the population of low birth weight preterm infants without focal parenchymal abnormalities revealed by conventional MR imaging. Other authors have reported conventional MR imaging abnormalities in 82% to 100% of patients with CP who underwent imaging at term or term-equivalent age (1, 22, 23). We plan to follow our patients with repeat imaging into childhood and adulthood to correlate their subsequent MR imaging findings with the early MR imaging and diffusion tensor imaging results.
Diffusion Tensor Imaging Findings
Diagnostic diffusion tensor imaging was performed for 63 (70%) of the 90 patients who had undergone normal conventional MR imaging. The major reasons for incomplete diffusion tensor imaging were twofold: 1) diffusion tensor imaging sequences were inadvertently excluded or not adequately acquired, and 2) patient motion artifact. The former factor was drastically improved during the first few months of the study as the imaging team became more accustomed to the diffusion tensor imaging sequence and proper imaging parameters. The second factor occurred unpredictably throughout the study. The diffusion tensor imaging sequence was always acquired at the end of the study, after the conventional T1-weighted, T2-weighted, fluid-attenuated inversion recovery, and gradient-echo images had been obtained. Because imaging was performed without patient sedation, images of infants who awoke before or during the diffusion tensor imaging sequences were prone to motion artifact. Patients who had diagnostic conventional images but motion-degraded diffusion tensor images were not returned for repeat diffusion tensor imaging.
In our study, images of premature infants were obtained near term-equivalent age. This has become the standard method for comparing patient subgroups, including preterm infants born at different gestational ages (13, 15). A number of these authors have reported ADC and FA data for preterm infants in the 25- to 40-week gestational age range; these data assist in the interpretation of diffusion tensor imaging values measured at term (13, 15, 24). Term-equivalent MR imaging of premature infants before discharge is also of practical importance because patients usually are stable and require less monitoring and cardiorespiratory support. MR imaging can also provide prognostic information and guide follow-up therapy (1).
Whole Brain Histogram Analysis
Histologic analysis of white matter abnormalities in infants with periventricular leukomalacia usually shows more diffuse injury than is shown by conventional MR imaging (25). As many as two-thirds of infants with CP have histologically diffuse white matter injury (26, 27). Despite these facts and despite the regional differences in white matter quantitative FA measurements described herein, whole brain ADC and FA analysis showed no measurable differences between patients with CP with abnormal neurologic outcomes and patients without CP or normal neurologic outcomes who had normal conventional MR imaging findings. Although a slight trend to lower ADC values was shown among patients with CP compared with normal patients, this did not achieve statistical significance. Intuitively, we would not expect lower ADC values in areas of abnormal development in the absence of recent parenchymal ischemia. It is possible that our patient population was not large enough to detect differences in whole brain tensor measurements with sufficient statistical power. A second factor to explain this finding is that the whole brain histograms include large areas of the brain that may be relatively spared and/or compensated in patients with CP with abnormal neurologic outcomes, including cortical and central gray matter. Third, because the patients studied all had normal or minimal abnormalities shown by conventional MR imaging, they may represent those infants with abnormal neurologic outcomes with the subtlest anatomic findings.
Region-of-Interest Analysis
Diffusion tensor imaging of preterm infants allows quantitative assessment of developmental white matter changes. Evidence exists for progressive maturation and organization of white matter tracts characterized by decreasing ADC and increasing FA values (13, 15), a trend that continues throughout childhood and adolescence (28, 29). In accordance with earlier maturation of posterior limb versus anterior limb of the internal capsule, we found higher FA values in the former versus the latter structure in our patients with normal neurologic outcomes. The corpus callosum and the posterior limb of the internal capsule showed the highest FA values, whereas the lowest values were found in cerebral white matter, again in accordance with stages of maturation of white matter in the brain. In neonates and infants, these findings have been attributed to premyelination changes in axonal diameter, cell membrane channels, and oligodendroglial organization (16).
Regarding regional diffusion properties, it has been shown that ADC values decrease as gestational age increases in the internal capsule and in the cerebral white matter (13, 15). In addition, preterm infants who undergo imaging at term-equivalent age show ADC values in the internal capsule comparable with full-term control infants but persistent high ADC in the cerebral white matter (13). This suggests that white matter maturation in premature infants differs quantitatively from in utero maturation. In our study, regional analyses showed a trend toward higher ADC values in the cerebral white matter and the splenium of corpus callosum in patients with abnormal neurologic outcomes. However, no significant differences among the clinical subgroups were found based on average ADC values in any other regions of interest. This is not surprising considering the findings presented by Huppi et al (14), who showed that ADC values in the periventricular white matter and the posterior limb of the internal capsule do not differ significantly in preterm infants with normal MR imaging compared with those infants with periventricular white matter injury who underwent imaging at term-equivalent age.
The evolution of FA values in preterm infants has also been described in the preterm and term-equivalent periods. Huppi et al (13) showed that preterm infants have lower FA values in the posterior limb of the internal capsule and cerebral white matter compared with term infants. They also found lower FA values in the posterior limb of the internal capsule and periventricular white matter in preterm infants with white matter injury versus control infants (14). Our analysis of average FA values showed a significant reduction in the white matter of the right posterior limb of the internal capsule in patients with CP with abnormal neurologic outcomes compared with patients with normal outcomes. A trend for significant lower FA values was also noted in the left posterior limb of the internal capsule bilaterally in normal patients with abnormal neurologic outcomes compared with normal neurologic outcomes. The range of FA values and the corresponding overlaps between preterm infants with normal and abnormal neurologic outcomes are presented in Table 3. Considering the small number of cases studied to date, we were unable to ascertain an FA threshold below which to predict abnormal outcome. However, the present study is designed to continue until the year 2005 to include a larger number of cases and to enable us to make receiver operating characteristic curves. Perhaps this will provide a sample sufficient to achieve predictive value measurements.
In the internal capsule, significantly decreased mean FA values in patients with abnormal neurologic outcomes (both with and without CP) suggest the presence of axonal injury, abnormal axonal development/myelination, or both (14). The posterior limbs of the internal capsules comprise the anatomic site of the cortical projections in the periventricular deep white matter, a common area of parenchymal injury in preterm infants with CP. It has been suggested that central white matter axonal injury can result in impaired development of white matter tracts distal to the injury (30), including diffusion tensor imaging abnormalities in parallel fiber white matter tracts along the affected pathway, which may represent wallerian degeneration (31). In particular, decreased regional FA measurements in the posterior limb of the internal capsule have been shown in patients with periventricular white matter abnormalities (14). In our study, significant reduction in the internal capsule FA values was observed in the absence of internal capsule and periventricular signal intensity abnormalities on conventional MR images of patients who subsequently were shown to have clinical neurologic abnormalities. A recent study (32) of two children who had been preterm infants with spastic quadriplegia secondary to periventricular leukomalacia who underwent imaging at 6 years of age showed substantial changes in FA on the 3D reconstruction of the posterior limbs of the internal capsules with no changes in the direct corticospinal tract per se. These data suggest involvement of corticothalamic, corticobasal ganglia, and corticoreticular projection abnormalities in patients with CP. These results are in support of our findings and emphasize the significance of posterior internal capsule involvement in the pathophysiology of neurologic abnormalities, including CP in preterm infants. Periventricular regions are well-known sites of white matter injury in premature infants based on imaging and histologic studies (22, 27, 33). Diffusion tensor imaging measures in these areas may provide an early measure of axonal microstructural abnormality, whereas subsequent imaging of children and adults can show evidence of diffuse or focal atrophy (4).
Our data shows significant reduction in mean FA bilaterally in the posterior limb of the internal capsule in patients with abnormal neurologic outcomes, although it was statistically significant only on the right side in patients with CP compared with infants with normal neurologic outcomes. A significant increase in the ipsilateral anterior limb of the internal capsule in patients with CP may represent increased myelination of the remaining internal capsule, a so-called status marmoratus. Both posterior and anterior limbs of the left internal capsule showed no differences between patients with CP and control patients. The laterality of our finding may partly be because two of 13 of our patients with CP or abnormal neurologic outcomes had right-sided subependymal signal intensity abnormalities shown by conventional MR imaging. This suggests that subependymal signal intensity abnormalities are neither sensitive nor specific for subsequent neurologic abnormalities and may, in some cases, serve as a marker for injury or maldevelopment of ipsilateral structures. In our study, 18 infants with such abnormalities had normal neurologic outcomes (see Table 2). Further diffusion tensor imaging studies of a larger group of patients with CP with subependymal low signal intensity abnormalities may further elucidate the clinical significance of this finding.
Our study showed a modest reduction of FA values in the cerebral white matter in patients with abnormal neurologic outcomes versus normal patients, but no significant differences were found in patients with CP. Despite that, periventricular white matter is a common site of signal intensity abnormality in preterm infants with CP with focal MR imaging findings (22). Several reasons may explain our observations. First, this was a small number of patients with CP with no significant focal abnormalities revealed by conventional MR imaging. Second, the cerebral white matter as visualized on axial view imaging typically includes mostly supraventricular periventricular white matter. The periatrial parietotemporal white matter, which is a common site of white matter injury, was underrepresented on the images we used for diffusion tensor imaging calculations of cerebral white matter. Third, our axial view 4-mm images typically provided a single section through the supraventricular cerebral white matter. This section might be prone to partial volume artifact from the underlying ventricles and the overlying cortex, which could average out potential statistically significant diffusion tensor imaging measures. Diffusion tensor imaging in the coronal plane in thinner sections in a larger group of patients with CP may prove more useful for characterization of the periatrial white matter injury.
Conclusion
In summary, diffusion tensor imaging with region-of-interest analysis is sensitive to regional white matter microstructural differences, which are significantly different in preterm infants with abnormal neurologic findings compared with those with normal neurologic outcomes. Regional differences in FA may allow for early detection of infants at risk for neurologic sequelae. Several groups have also used diffusion tensor imaging to examine microstructural differences in patients with more subtle neurodevelopmental abnormalities, including intelligence and developmental delay (34, 35). These findings show the tremendous potential of diffusion tensor imaging to further our understanding of microstructural brain abnormalities associated with pathologic abnormalities that are often underestimated by, or completely occult on, conventional images. More importantly, in the case of CP in preterm infants, early detection and evaluation of injury for infants at high risk may become particularly pertinent as early interventions are further developed (26, 36, 37). In conclusion, although conventional MR imaging is of proved value for diagnosis and prognostic information regarding preterm infants with suspected neurologic injury abnormalities, the use of additional diffusion tensor imaging sequences may increase the sensitivity and specificity of MR imaging for predicting neurologic outcome. This may provide further quantitative information regarding the microstructural anatomy of the injury. This diagnostic approach to predicting later neurologic abnormalities may become the basis for planning future interventional clinical studies to improve outcomes.
Footnotes
This work was supported in part by the National Institute of Neurological Disorders and Stroke grant R21 NS40374 and a National Institutes of Health grant to the General Clinical Research Center and the Packard Foundation (Lucile Packard Children’s Hospital Innovation in Patient Care).
Y.A. and M.M. contributed equally.
References
- Received December 6, 2002.
- Accepted after revision April 14, 2003.
- Copyright © American Society of Neuroradiology