Abstract
BACKGROUND AND PURPOSE: Presbycusis is the most common sensory deficit in the aging population. A recent study reported using a DTI-based tractography technique to identify a lack of integrity in a portion of the auditory pathway in patients with presbycusis. The aim of our study was to investigate the white matter pathology of patients with presbycusis by using a voxel-based analysis that is highly sensitive to local intensity changes in DTI data.
MATERIALS AND METHODS: Fifteen patients with presbycusis and 14 age- and sex-matched healthy controls were scanned on a 3T scanner. Fractional anisotropy, mean diffusivity, axial diffusivity, and radial diffusivity were obtained from the DTI data. Intergroup statistics were implemented on these measurements, which were transformed to Montreal Neurological Institute coordinates by using a nonrigid image registration method called large deformation diffeomorphic metric mapping.
RESULTS: Increased axial diffusivity, radial diffusivity, and mean diffusivity and decreased fractional anisotropy were found near the right-side hearing-related areas in patients with presbycusis. Increased radial diffusivity and mean diffusivity were also found near a language-related area (Broca area) in patients with presbycusis.
CONCLUSIONS: Our findings could be important for exploring reliable imaging evidence of presbycusis and could complement an ROI-based approach.
ABBREVIATIONS:
- DA
- axial diffusivity
- dB HL
- decibel hearing level
- DR
- radial diffusivity
- FA
- fractional anisotropy
- MD
- mean diffusivity
- PTA
- pure-tone average
Age-related hearing loss, also known as presbycusis, is the most common sensory deficit in the aging population. Presbycusis is usually characterized by progressive hearing loss at high frequencies, which are particularly important for speech recognition. Hypofunction of the inner ear is the main reason for the peripheral component of presbycusis.1 However, poor speech discrimination and deteriorated temporal sound processing reflect a possible central component of presbycusis.2,3 Moreover, many animal studies have showed the existence of a central component of presbycusis.4,5
Recent studies of multiple MR imaging modalities have demonstrated their capabilities of offering reliable imaging markers for recognizing presbycusis. In a structural MR imaging study, the volume and surface area were decreased in the auditory cortex areas of patients with presbycusis compared with young healthy controls.6 In a functional MR imaging study, patients with presbycusis showed higher blood oxygen level–dependent activation in response to acoustic stimuli in the temporal lobes compared with young healthy controls.7
The aforementioned imaging markers were mainly explored in gray matter, whereas white matter integrity, as one of the most sensitive indicators of axon damage or demyelination, requires further study. A widely used technique, DTI, has been considered as the most effective method for characterizing white matter organization. Multiple scalar measurements, including fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (DA), and radial diffusivity (DR), can be calculated from DTI data through tensor calculation. These measurements are useful indicators for characterizing various types of pathology in the human brain.
Many DTI studies have reported changes in the auditory pathway and auditory cortex in patients with sensorineural hearing loss.8,9 To our knowledge, only 1 study has reported a lack of white matter integrity in patients with mild presbycusis by applying an intergroup statistic on the ROI-based features, which was in the form of reconstructed auditory tracts.6 Besides that, the study showed no obvious differences in any measured DTI parameters between mild presbycusis and expressed presbycusis. Although promising, a more reliable imaging marker of presbycusis could be established by designing more sophisticated experiments. First, the reconstructed auditory pathway was partly obscured by the optic radiation. This so-called “fiber-crossing issue” could be mitigated by using probabilistic tractography; however, the “gold standard” for validating the tractography, especially in the fiber-crossing area, has not been established. In addition, though using the average characteristic with an ROI is an effective manner of dimensional reduction and noise filtering,10 a limitation is its reduced sensitivity to localized anatomic alterations that only affect parts of a predefined structure.11 Therefore, we used a voxel-based analysis, which might be considered as an alternative way to explore the imaging makers of presbycusis in the white matter.
Materials and Methods
Patients
This study was approved by the Shandong University institutional review board, and each participant provided informed consent. Fifteen patients with mild presbycusis (presbycusis group, 5 men and 10 women; mean age, 63.2 ± 2.6 years) who visited the department of otolaryngology of the central hospital of Jinan city were recruited in this study (Table). Hearing loss was defined as a speech-frequency pure-tone average (PTA) of thresholds at 0.5, 1, 2, and 4 kHz (air conduction) in the better-hearing ear, per the definition of hearing loss adjudicated by the World Health Organization. The PTA value of 25 decibel hearing level (dB HL) was accepted as the normal hearing threshold limit.12 Inclusion criteria were mild hearing loss (defined as 25 dB HL < PTA ≤ 40 dB HL in the better-hearing ear) and age ≥60 years. Exclusion criteria were ear diseases that affect hearing thresholds and sensorineural hearing losses other than presbycusis; history of otologic surgery, ototoxic drug therapy, noise exposure, or hearing aid use; asymmetric hearing loss with a difference in air conduction thresholds exceeding 20 dB in at least 2 frequencies between 0.5, 1, 2, and 4 KHz; conductive hearing loss (the mean air-bone differences at 0.5, 1, 2, and 4 KHz) >10 dB in 1 or both ears; and tinnitus, head trauma, lesions of the facial nerve, disorders of the cervical spine, or neurologic or psychiatric disease. The complete list of exclusion criteria was previously described.13
Demographic and auditory function of the presbycusis and control groups
Fourteen age- and sex-matched healthy controls (control group, 6 men and 8 women; mean age, 62.4 ± 2.0 years; PTA ≤25 dB HL in the better-hearing ear) were recruited for this study. All controls were in good health and had no history of neurologic or psychiatric disease. Healthy controls were matched for education levels (Table).
None of the subjects were music professionals. All subjects were right-handed according to Li's Handedness Inventory, which was designed for Chinese populations.14,15
Assessment of Auditory Function
An otoscopic examination was performed in all patients to remove cerumen and confirm the presence of an intact tympanic membrane. The hearing abilities of all patients were evaluated by using pure-tone audiometry and tympanometry. The pure-tone audiometry was performed with a Madsen Electronics Midimate 622 Clinical/Diagnostic Audiometer (GN Otometrics, Taastrup, Denmark) coupled with Telephonics TDH-39P headphones (Telephonics, Farmingdale, New York). Bone conduction was measured at 0.25, 0.5, 1, 2, and 4 KHz, and air conduction was measured at 0.125, 0.25, 0.5, 1, 2, 4, and 8 KHz. Hearing thresholds were detected with a resolution of 5 decibel steps. The PTA values of all patients' ears were calculated. The tympanometry was performed with a Madsen Electronics Zodiac 901 Middle Ear Analyzer (GN Otometrics) to confirm optimal middle-ear conditions.
DTI and Data Processing
All patients were scanned on a 3T scanner (Achieva TX; Philips Healthcare, Best, the Netherlands) using an 8-channel phased-array head coil as a receiver. The DTI dataset was acquired with a multisection, single-shot, EPI, spin-echo sequence (TR/TE = 6281/67 ms; sensitivity encoding factor = 2.5). Sixty-five transverse sections were acquired parallel to the line connecting the anterior commissure to the posterior commissure with no section gap and 2.2-mm nominal isotropic resolution. Diffusion weighting was applied along 32 directions with a b-value of 700 seconds/mm2. Five minimally weighted images (5 B0 with b=33 seconds/mm2) were acquired and averaged on the scanner as part of each DTI dataset.
The raw diffusion-weighted images were first coregistered to the B0 images and corrected for eddy current and patient motion with affine transformation by using Automated Image Registration (http://bishopw.loni.ucla.edu/air5/).16 The 6 elements of the diffusion tensor were calculated for each pixel with multivariate linear fitting by using DTIStudio (Johns Hopkins University, Baltimore, Maryland).17 After diagonalization, 3 eigenvalues and eigenvectors, as well as FA, MD, DA, and DR, were obtained. A 2-step image transformation was used to warp the individual data to a standard template, JHU-MNI-ss (Johns Hopkins University, www.mristudio.org) in the ICBM-152/ICBM-DTI-81 space. First, affine transformation was used to globally adjust the brain position, rotation, and size. Next, a nonlinear transformation with large deformation diffeomorphic metric mapping18 was applied to accurately warp the local structures. The dual-contrast large deformation diffeomorphic metric mapping, in which both the B0 image and the FA map were applied simultaneously, was used. These procedures are reciprocal and provide forward (subject to atlas) and backward (atlas to subject) transformation matrices. FA, MD, DA, and DR images then were transformed to template space by using the forward matrix. After the registration, all images were smoothed using statistical parametric mapping by setting the full width at half maximum = [4 4 4].
Statistical Analysis
The 2-tailed Student t test was performed to test for differences in age, education levels, and PTA between patients with presbycusis and healthy controls. The differences in sex were evaluated by a 2-tailed Pearson χ2 test. The threshold of significance was set at P = .05. Statistical analyses were performed by using the SPSS version 18.0 software package (IBM, Armonk, New York).
Intergroup statistics were implemented (voxel by voxel) to several DTI indices, including FA, MD, DA, and DR, which had been transformed to the standard space. The results were obtained from SPM8 (http://www.fil.ion.ucl.ac.uk/spm/) by the application of multiple comparisons and the setting of thresholds of P < .001 (uncorrected) at voxel level and P < .05 (family-wise error–corrected) at cluster level. The minimum cluster size was 100 voxels. The results were visualized by using a Matlab (MathWorks, Natick, Massachusetts) toolbox named xjview (http://www.alivelearn.net/xjview).
Results
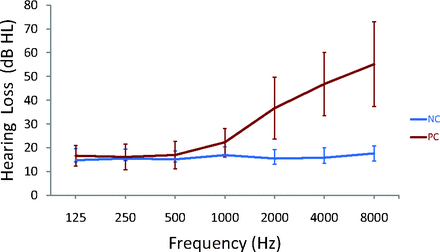
All patients had a type-A curve on tympanometry, which indicated normal middle-ear function. There was no significant difference in PTA between the left and right ears in the presbycusis group (P = .49) or the control group (P = .10); therefore, the thresholds of both ears were averaged in each group. The PTA was significantly higher in patients with presbycusis than in healthy patients (P < .001 [Table]). In the control group, the mean hearing thresholds were <20 dB HL at all frequencies; in the presbycusis group, the mean hearing thresholds were >20 dB HL at 1 kHz and reached 46.8 dB HL at 4 kHz and 55.2 dB HL at 8 kHz (Fig 1).
Results of pure-tone audiometry from 125 Hz to 8000 Hz (air conduction). Hearing thresholds from both ears are averaged (mean ± standard deviation) in the presbycusis (PC) and healthy control (NC) groups.
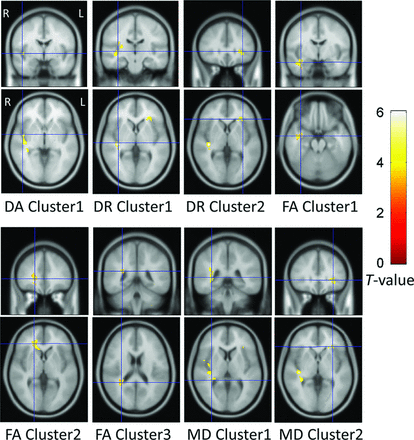
The results of voxel-based analyses are shown in Fig 2. The presbycusis group showed increased DA near the right-side Heschl gyrus and increased DR near the right-side Heschl gyrus and the left-side inferior frontal gyrus. The presbycusis group showed decreased FA near the right-side angular gyrus and inferior frontal gyrus and decreased FA near the right-side temporal pole and middle temporal gyrus, which were partly overlapped with acoustic radiation. The presbycusis group also showed increased MD near the right-side Heschl gyrus and the left-side inferior frontal gyrus. The On-line Table specifies the above-mentioned areas distributed in the brain image as voxel clusters with detailed information, including the peak coordinates in the Montreal Neurological Institute space, cluster-level P value, and peak T-value of each cluster.
Voxel-based analysis results. The DA, DR, and MD were significantly increased and the FA was significantly decreased in the presbycusis group compared with the healthy control group (P < .001 at voxel level, uncorrected; P < .05 at cluster level, family-wise error–corrected). Each cluster contains more than 100 adjacency voxels that show significant variation of those diffusion parameters. For example, DA cluster 1 denotes the first group of voxels that show significant intergroup difference of DA, whereas DR cluster 1 denotes the first group of voxels that show significant intergroup difference of DR. The significant voxels are displayed with their T-values. The ascending T-values are shown from darkness to brightness. Each panel displays a single cluster with its location indicated by a blue section marker.
Discussion
Our study demonstrated altered DA, DR, FA, and MD near the right-side hearing-related areas of patients with presbycusis compared with age- and sex-matched healthy controls. Increased DR and MD were also observed in a language-related area (Broca area) of patients with presbycusis. To our knowledge, this is the first in vivo demonstration of presbycusis-related changes over the entire brain in a DTI fashion by using a voxel-based analysis.
The scalar measurements of DTI were capable of characterizing various white matter pathologies. Among all of these measurements, FA was sensitive to various microstructural changes19; MD was considered the inverse measure of the membrane attenuation and fluid viscosity20; DA was considered highly sensitive to axonal degeneration; and DR was sensitive to demyelination.21 DTI technique was introduced to study the macroscopic architecture of white matter. However, several animal studies have shown changes of DTI parameters, including MD, DA, and DR, in gray matter areas because of neuron loss. FA did not account for those contributions because the dendrites are only partially coherently organized and the axons are not homogeneously distributed and might be myelinated or unmyelinated in gray matter.22,23 When overlaid to the statistical parametric mapping–integrated T1 template, the changes were seen mostly within the subcortical white matter areas with a small portion of voxels overlapped with cortex. Because it is difficult to separate the white matter and gray matter with DTI contrast, the results are elaborated with “gyrus” (which contain both subcortical white matter and cortex) as its units. In our study, increased DA, DR, and MD were found near the right-side Heschl gyrus in patients with presbycusis; decreased FA was also seen in white matter areas that partly overlapped with the right-side acoustic radiation. To further examine the functional representation of the clusters shown in the On-line Table, we overlaid the Brodmann atlas to our results and found that changes overlapped with superior temporal gyrus and angular gyrus were close to Brodmann area 41, which refers to the auditory cortex. These results could indicate a dysfunctional auditory pathway in presbycusis. This is consistent with previous studies where the DTI-based parameters were changed at the Heschl gyrus, superior temporal gyrus, lateral lemniscus, auditory radiation, and inferior colliculus in patients with sensorineural hearing loss.9,24,25 Two possible explanations for the alteration shown in the right-side hearing-related areas are as follows: 1) Presbycusis is most often characterized by a decline in frequency audibility toward high frequencies, which is crucial for pitch perception. One study has demonstrated that the right hemisphere was the dominant hemisphere involved in pitch perception26; and 2) Another study found a clear rightward asymmetry in the white matter under the auditory cortex in patients with presbycusis.6
To our knowledge, only 1 study has used a DTI-based technique to investigate microstructural changes in the white matter of patients with presbycusis.6 In that study, the DA value was increased in the right-side auditory pathway of patients with mild presbycusis compared with young healthy controls. Therefore, the altered integrity of the auditory pathway might be mainly caused by aging. In our study, age-matched healthy patients served as controls, so we determined the altered integrity of the white matter in the right-side Heschl gyrus is mainly driven by hearing loss. However, there were no differences in any measured DTI parameters between patients with mild presbycusis and expressed presbycusis in the previous study.6 Except for the selection of ROI sizes (the minimal size of an ROI is a single voxel), as mentioned in the introduction, the contradiction in results might be explained by differences in the patient populations.
Apart from the findings along the auditory pathway, our results also showed changes in a language-related area within the inferior frontal gyrus (near the Broca area). This finding is consistent with previous studies: decreased audibility in patients with presbycusis influences speech processing, especially in noisy environments.3 The inability to clearly detect temporal cues is a possible reason for decreased speech understanding in patients with presbycusis.2,27 The processing of temporal cues, which mainly occurs in the auditory cortex,28 might be influenced by age-related changed inhibition.29,30 Gamma-aminobutyric acid is the main inhibitory neurotransmitter in the central auditory system. One MR spectroscopy study demonstrated that gamma-aminobutyric acid concentrations were reduced in the auditory cortex of patients with presbycusis.31 The inhibition-deficit hypothesis is also considered the reason for the decline in several other cognitive domains, such as reading.32 Altered DTI parameters were also found near the insula in patients with presbycusis. This is consistent with functional imaging studies that have shown that the insula plays an important role in auditory processing.33
Voxel-based analysis, which is capable of localizing very subtle anatomic changes, suffers from 2 major limitations. First, it searches for the group differences in a hypothesis-independent way.34 However, disease-related changes can be distributed in a complex fashion. Therefore, the voxel-based analysis is not sensitive for the detection of small and widely distributed changes in image intensities. Second, voxel-based analysis is sensitive to registration accuracy, which is not reliable enough when based on DTI. This is because DTI does not contain enough structural information compared with T1WI. The registration errors could be reduced by using a more sophisticated approach to further improve accuracy of voxel-based analysis. In addition, DTI is itself limited because it often has to pay a penalty of a higher SNR and higher sensitivity to measurement artifacts such as patient motion and eddy current.35,36 Finally, our study was further limited by the small sample size; hence, it should be viewed as a preliminary study and requires confirmation in larger samples.
Conclusions
Using a voxel-based analysis, this study revealed presbycusis-related changes of DTI parameters along the auditory pathway and a language-related area. Our findings could be important for exploring more reliable imaging evidence of presbycusis and could complement the ROI-based approach or studies by using different patient populations.
Footnotes
W. Ma and M. Li contributed equally to this work.
This project was supported by the National Natural Science Foundation of China (grants 81171380/H1807 and 81371534), Shandong Provincial Natural Science Foundation of China (grant BS2015YY003), and Shandong Provincial Medical and Healthy Technology Development Program of China (grant 2015WS0176).
Indicates open access to non-subscribers at www.ajnr.org
References
- Received January 28, 2016.
- Accepted after revision May 9, 2016.
- © 2016 by American Journal of Neuroradiology