Abstract
SUMMARY: Embolization of the middle meningeal artery has gained substantial interest as a therapy for chronic subdural hematomas. For the results of the currently running chronic subdural hematoma trials to inform clinical practice, sufficient accuracy and matching definitions are necessary. We summarized the current practice in chronic subdural hematoma evaluation and derived suggestions on reporting standards using the {Nested} Knowledge AutoLit living review platform. On the basis of the most commonly reported data elements, we suggested a set of standardized image-based study end points for chronic subdural hematoma evaluation for future trials. The measurement methods and reporting standards as proposed in this article have been derived from published best practices and are endorsed by the European Society of Minimally Invasive Neurological Therapy’s research committee. The standardization of radiologic outcome measures and measurement techniques in chronic subdural hematoma embolization trials would increase the impact and implication of each trial as well as facilitate data pooling for increased statistical power and, therefore, translation to clinical practice.
ABBREVIATIONS:
- cSDH
- chronic subdural hematoma
- MLS
- midline-shift
- MLS-M
- MLS versus midline or displacement perpendicular to the midline
- MLS-T
- MLS transverse
- MMA
- middle meningeal artery
- NK
- {Nested} Knowledge
- SDH
- subdural hematoma
Chronic subdural hematoma (cSDH) is a frequently occurring pathology in daily neurosurgical practice, with increasing frequency as the population ages.1 However, there is still a relative lack of high-quality evidence at many decision points in the treatment algorithm of the typical patient with cSDH, which is far from optimized.2 Despite numerous studies investigating the management of cSDH, questions about the choice of surgical technique, adjuvant therapies, and postoperative care remain unanswered. Many of the studies published in the literature report heterogeneous baseline data, using variable terminology and definitions of operative technique, and evaluate disparate outcome measures.3 Even cSDH lacks a universally accepted definition.4
In recent years, middle meningeal artery (MMA) embolization has emerged as a new and promising therapy option for cSDH.5⇓-7 Numerous clinical trials evaluating the safety and efficacy of this new treatment method have recently been initiated, and some have already published their results.7 A systematic review of 96 studies examining clinical outcomes in patients with cSDH revealed that 39% of the studies used a radiologic outcome measure generally based on a postoperative CT scan. However, these radiologic outcome measures were highly heterogeneous, as was the timing of the scans.3 Furthermore, there seems to be a lack of consensus on how to determine a specific radiologic outcome measure. For example, various techniques on how to measure subdural hematoma (SDH) thickness and volume or even midline shift (MLS) exist.8⇓-10 These techniques pose a significant barrier to establishing an evidence-based approach for the management of cSDH, as stated in published meta-analyses that have sought to elucidate the optimal treatment options for cSDH.3,6,11 To overcome these barriers and enable cross-study evaluation of the efficacy of MMA embolization, and other cSDH treatments, the development of standardized outcome measures is needed, which should subsequentially be reported by all clinical studies and trials concerning a specific disease state.
In this article, we describe and discuss the heterogeneity of radiologic outcome measures for clinical trials on MMA embolization for cSDH. To emphasize the relevance of a common definition and selection of radiographically defined parameters, we completed a review of radiologic outcomes and inclusion/exclusion criteria in active cSDH trials. Furthermore, we propose a potential standardized methodology for defining and measuring radiologic outcomes of cSDH, as well as how and when to report them. Because published research on the evaluation of the accuracy and reliability of specific radiologic outcome measures such as cSDH volume, width, and MLS specifically in cSDH is sparse, the measurement methods and reporting standards as proposed in this article have been derived from the best published practices and are endorsed by European Society of Minimally Invasive Neurological Therapy’s research committee.
MATERIALS AND METHODS
Clinical Trials of cSDH Embolization
We performed a literature search of ClinicalTrials.gov to identify currently active or complete and unpublished trials on MMA embolization in patients with cSDH. ClinicalTrials.gov was searched for the terms Chronic AND Subdural AND (hematoma OR haematoma OR hemorrhage OR haemorrhage OR bleeding) AND (embolization OR embolization) on August 7, 2021. The review of these clinical trials was then conducted through the AutoLit platform (Nested Knowledge [NK]; https://nested-knowledge.com).12
All interventions used and radiologic outcome data elements as well as image-based inclusion or exclusion criteria were tagged by using the AutoLit tagging feature. Included studies were scanned for the predetermined radiologic outcomes with the related definitions and follow-up time points, and a unique tag was created for each data element found on the basis of a full-protocol review, as relevant. All tagging was completed by 1 author and quality-controlled by an independent author.
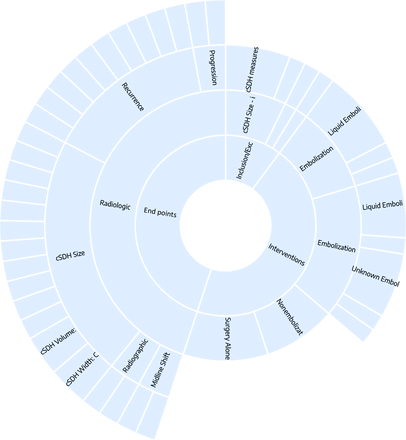
Qualitative synthesis on the frequency of study design types and data elements was graphically presented in the form of a sunburst diagram on the Synthesis feature (NK) after the completion of tagging different studies. Each section represented a tag that was applied across trials, and frequency could be determined by the platform on the basis of the number of studies that had that tag (Fig 1; for interactive version, see https://nested-knowledge.com/nest/qualitative/461). After quantitative summary data were extracted from the NK Qualitative Synthesis feature, the appropriate tables and figures were created using Microsoft-based data presentation software (Microsoft Excel and PowerPoint).
Sunburst diagram of data elements in the NK nest for this study. Clicking on each data element outputs a frequency of the tag associated with it, as well as frequently co-occurring tags. See https://nested-knowledge.com/nest/qualitative/461 for an interactive version of this figure.
RESULTS
Study Characteristics
As of August 7, 2021, fifteen studies, of these 12 randomized controlled trials, relating to embolization of the MMA of cSDH had been identified on clinicaltrials.gov. All of these 15 studies used image-based measures as end points. The main imaging-based outcome data elements and inclusion/exclusion criteria were based on measures of the following: cSDH size, MLS, radiographic resolution, radiographic recurrence, and radiographic progression. The most frequent radiologic outcome measures identified were cSDH size (11/15, 73.3%) and cSDH recurrence (9/15, 60%).
Five of 15 (33.3%) trials also reported radiologic measurements as inclusion or exclusion criteria. The most frequently identified in-/exclusion criterion was cSDH width > 10 mm (in 4 of 5). However, some of these criteria may evolve as discussions among the trial investigators continue. Details on the radiologic measures are listed in the Online Supplemental Data.
In addition, the planned follow-up periods and intervals of the trials were highly heterogeneous: Periods of follow-up ranged from 6 weeks to 12 months, with intervals between visits ranging from 2 weeks to 6 months.
SDH Width and Volume Measurement
As in Online Supplemental Data the determination of cSDH width and volume is an essential factor for cSDH treatment monitoring and the definition of radiologic recurrence or progression.
However, for both variables, a number of different measurement techniques have been published and compared. For cSDH volume estimation, manual computer-assisted volumetric analysis is considered the gold standard. To facilitate volume measurement in cSDH, a simple bedside estimation method, known as the ABC/2 method, was proposed and validated for use in the measurement of acute and cSDH volume.13⇓-15 This method is based on the mathematic formula for the volume of an ellipsoid, 4/3π × (A/2) × (B/2) × (C/2), where A, B, and C represent the 3 diameters of the ellipsoid commonly measured on the axial plane. If π is estimated to be 3, the formula simplifies to ABC/2.16⇓-18 To our knowledge, only 3 published studies explicitly evaluated this volumetric measurement technique specifically in patients with cSDH.14,15,19 In addition, the study by Gebel et al13 involved patients with acute subdural hematomas as well as chronic ones.
Overall, the assessed volume measurement techniques showed a high correlation with the criterion standard in patients with acute as well as cSDH.13⇓-15 Sucu et al15 compared 5 different ABC/2-based volume measurement formulas to identify the formula that provided the most accurate estimation of hematoma volume compared with the criterion standard. Although all 5 formulas showed excellent correlation with the criterion standard, the ABC/2 method with the measurement of maximum width and length, which are not necessarily on the same section, achieved the highest correlation coefficient. Won et al14 found a correlation between ABC/2 and computer-assisted values with an R2 of 0.93 when evaluating 100 cSDHs in 82 patients. This group used the section with the maximum length to determine maximum width (taken perpendicular to the length). For determination of hematoma depth, the number of slices with visible hematoma multiplied by section thickness was performed in all 3 studies.13⇓-15
To elucidate some of the shortcomings and problems arising from the above-mentioned techniques, we applied some of these width-measurement methods to specific patients with unevenly shaped hematomas (Fig 2C). These cases illustrate that accurately following the given measurement techniques may lead to measurements that do not seem to correspond to hematoma width as we would define it according to the underlying formula of the radius of an ellipsoid-shaped body. As demonstrated in Fig 2, inaccurate width measurements are particularly likely to occur in hematomas that are close to the vertex and those that are irregularly (ie, not crescent) shaped. Indeed, the further removed the cSDH collection is from an ellipsoid shape, the less accurate the ABC/2 formula is.20 According to a study by Manickam et al19 that measured the proximity to an ellipsoid shape using 3D simulations, most cSDHs demonstrated highly irregular morphology, and only a very few (9%) remotely conformed to ellipsoid geometric morphology.
The effect of SDH morphology on volume calculation using the ABC/2. The formula is derived by assuming a crescent shape (the difference between a large ellipsoid and a small ellipsoid, both of which are cut in half). A and B, L = length; W = width, difference between ellipsoids = Wa, Wb; C, Thickness (not shown) (L and C are the same for both ellipsoids). The formula thus reduces to volume of crescent-shaped cSDH = (4/3 π LWaC – 4/3 π LWbC) / 2 = (LWaC – LWbC)/ 2 = LWC/ 2 (= ABC/2). A, The assumed crescent shape allows accurate calculation due to the way the ABC/2 formula is derived (right panel). B, When the SDH is irregular, however, the ABC/2 loses accuracy and can lead to overestimation of the true volume. C, cSDH maximum width perpendicular to the maximum length marked in specific patients with unevenly shaped hematomas. Patient 1: width of the subdural hematoma measured on a section close to vertex (W) is greater than it actually is. Patient 2: inhomogeneous convex- and concave-shaped hematomas. Maximum width (W2) measurement is diagonal and not accurate. W1 would be more accurate in this case. Patient 3: maximum width measured perpendicular to length but slices hematoma diagonally.
Above the superior temporal line, axial CT slices are no longer perpendicular to the cranium or cSDH (Fig 2C, patient 1); rather, they run obliquely because of the curvature of the cranial vault. Therefore, the width of the cSDH measured on a section close to vertex is greater than it actually is. Furthermore, because of their chronic nature and traction of developing membranes, cSDHs are not always symmetrically crescent-shaped; they may appear as asymmetric shapes, such as a comma, pear, or lens on axial CT slices (Fig 2C, patients 2 and 3). As a result, whenever possible, computer-assisted volumetric analysis should be applied, especially in studies in which longitudinal analyses are performed. Most CT scanners today can produce axial slices of 0.625-mm thickness, generating relatively isometric voxels of 0.625 × 0.5 × 0.5 mm. These can be reconstructed in the coronal plane for improved accuracy of measurement.
Selection of radiologic measurement techniques requires both high accuracy and harmonization across studies to provide meaningful comparative data. Thus, even though most ABC/2 volume measurement techniques show a high correlation with the criterion standard,13⇓-15 a designated standard measurement technique needs to be defined to ensure comparability among clinical studies.
Another problem lies in the lack of validation of certain methodologies with respect to specific situations. For example, the question of the cSDH width-measurement technique most suitable to reliably detect changes in cSDH size, especially after therapy, remains currently unanswered. However, if cSDH width is reported, details on the assessment technique should be described and measurements should be reported in combination with cSDH volume.
Only a few studies address problems regarding cSDH segmentation. Sucu et al,15 for example, excluded 6 of 28 patients with cSDH because it was not possible to differentiate the isodense hematoma from the brain parenchyma on CT scans. Certainly, the frequency of this problem may also be dependent on the image contrast/quality produced by a particular CT protocol on a particular model. Therefore, in such scenarios, signs of mass effect, such as MLS and local cortical flattening, can be evaluated to compare cSDH sizes. However, how to include these cases in trials or studies in which accurate measurements of hematoma size and volume are compared pre- and postoperatively remains unclear. Nevertheless, such scenarios should be reported and described.
MLS
Another frequent outcome measure in cSDH studies is MLS, a sign of a space-occupying effect. Besides cisternal compression and sulcal flattening, MLS is an important indicator of mass effect and can help determine the need for surgical intervention.9
Different measurement techniques for the estimation of MLS have been published.8,9,21 There are 2 possible measurement techniques (MLS transverse: displacement relative to the tabula interna in relation to the width of the intracranial space [MLS-T] and MLS versus midline or displacement perpendicular to ideal midline [MLS-M]) that can each be combined with either a specific predefined anatomic measurement location (which also indirectly predefines the section and location of the measurement) or the identification of the location with the estimated largest MLS (which can be a different section and location in each patient). MLS estimation in cSDH according to these techniques can lead to very different measurements.21 Overall, the septum pellucidum seems to be the structure that is more sensitive to the space-occupying effect of intracranial masses. Variations, especially when longitudinal studies are analyzed, might also be dependent on section thickness and patient position or image reconstruction. Measurement of MLS-M might be a more reliable estimate and has shown high interobserver agreement.22 Moreover, determining the midline is easier than determining the width of the intracranial space, especially if the patient is not perfectly aligned during CT examination or if the skull is asymmetric, deformed, or has been removed by surgery or trauma, which is also of high relevance in studies including pre- and postoperative scans of patients with cSDH. So far, no study has systematically compared MLS estimations using the aforementioned measurement techniques in patients with cSDH and their specific intrarater and interrater variability.
On the basis of the currently available information, we propose the following MLS measurements in future cSDH therapy studies:
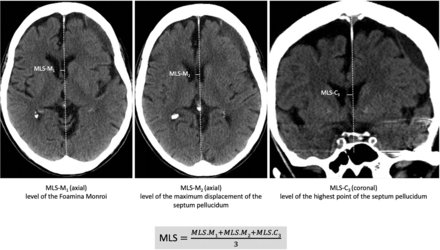
As MLS-M, ie, perpendicular to the midline joining the most anterior and posterior visible points on the falx, especially in studies including patients undergoing an operation (to increase pre- and postoperative comparability and decrease the effects of asymmetry of the skull)
If available, measurements should be conducted on axial and coronal slices
On axial slices, measurements of the maximum at the level and location of the foramen of Monro and as the maximum displacement of the septum pellucidum relative to the midline should be taken (to increase sensitivity for hematomas located at the convexity)
On coronal slices, measurements of the maximum MLS of the septum pellucidum at the level of the highest point of the septum should be taken (to decrease the effects of slice thickness and patient position or image reconstruction)
Overall, MLS should then be determined as the mean of these 3 measurements (or 2 in cases in which coronal reconstructions are missing).
Examples of the described MLS measurements are shown in Fig 3.
Proposed MLS measurement as the mean of maximum at the level and location of the foramen of Monro (MLS-M1), the maximum displacement of the septum pellucidum relative to the midline (MLS-M2), and the maximum MLS of the septum pellucidum at the level of the highest point of the septum on coronal slices (MLS-C3). Overall MLS is determined as the mean of these 3 measurements (or 2 in cases in which coronal reconstructions are missing). MLS-M indicates axial MLS perpendicular to the midline; MLS-C, coronal MLS perpendicular to the midline.
Patient presentation may also impact the level to which MLS indicates cSDH size and mass effect. Most notably, bilateral cSDH is common. When it occurs, the midline is pushed back to its normal position, making the MLS less useful in such patients. Atrophy is another factor influencing the degree of MLS. Therefore, other imaging features and clinical information must be taken into account to adequately evaluate the mass effect.10
Measuring postoperative MLS alone probably plays a smaller role in cSDH because clinical improvement can be achieved with partial evacuation, which could result in residual cSDH and MLS.10,23 Therefore, the MLS should always be assessed in conjunction with other parameters such as clinical information and cSDH volume. Nonetheless, we strongly encourage authors to provide a detailed description of the algorithms/measurement techniques used when reporting cSDH width, volume, MLS, or other quantitative radiologic measures. A summary of expert suggestions for measurement performance in cSDH is provided in Table 1.
Expert suggestions for radiologic measurements of cSDH for volume, width, MLS, and reporting
Follow-up Image Evaluation
Postoperative image findings and characteristics differ from those of preoperative examinations. Intracranial air collections, removal or deformation of the skull, and changes in hematoma shape and composition represent some of the issues that can complicate comparison of pre- and postoperative radiologic measurements.
Embolization of the meningeal artery is currently performed either as a primary therapeutic option in patients with cSDH or as a secondary measure when persistent or recurrent hematoma occurs following an operation. Therefore, these differences may also be relevant for a number of radiologic features obtained in the current embolization trials7 and should be carefully addressed, particularly with respect to longitudinal observations and treatment monitoring. Most studies include radiologic hematoma recurrence or progression as their primary or secondary end points (Table 1).
Hence, strict definitions and standardization of image analyses should be mandatory. However, to our knowledge, there are currently no studies specifically evaluating the applicability and accuracy of the above-mentioned measurement techniques in patients with cSDH after an operation, when early postoperative changes are still visible and the brain has not yet recovered and unfolded to its full extent. Therefore, in such cases, authors should provide detailed reports on whether air collections were included in volumetric measurements or how they obtained measurements if the skull had been removed or deformed.
Follow-up Period and Intervals for cSDH Embolization Studies
Standardization of follow-up periods and intervals of the cSDH embolization trials would be advantageous in that it would increase the overall value, validity, and significance of each individual clinical trial and enable joint analyses.
After surgical evacuation, routine follow-up CTs do not seem to provide any benefit over CTs performed only in patients with clinical deterioration or persisting neurologic deficits with respect to good clinical outcome.24 In addition, patients who were followed clinically without routine follow-up CT had fewer repeat surgeries—and this link may be causative, meaning that differential radiologic follow-up strategies are, in fact, influencing the data on reoperation and rendering studies yet more noncomparable in methods and outcomes.
Thus, in cSDH embolization studies, possible complications may occur during the postoperative period, such as infarction or new intracranial hemorrhage, for which the window of occurrence is not well-defined. In addition, radiologic progression is often stated as a primary or secondary outcome measure, and dictates whether additional surgical evacuation is performed.25 Furthermore, cSDH embolization might also be performed in combination with surgical evacuation therapy and is sometimes even performed in asymptomatic patients.26 Therefore, only performing posttreatment follow-up in case of clinical deterioration would be ineffective. However, currently, there are no studies explicitly evaluating the benefit of repeat and early follow-up CTs and the use of radiologic progression as the outcome parameter in cSDH embolization studies.
We, therefore, suggest a common imaging and clinical evaluation protocol for the application in cSDH embolization trials with a follow-up of 180 days (6 months).
To detect possible treatment-related complications such as ischemic infarction or new intracranial hemorrhage and to provide a postoperative reference for further comparisons, we suggest an early follow-up scan after 24 hours (1 day).
In addition, we suggest performing 2 additional CT scans because in contrast to surgical evacuation, embolization therapy does not result in an immediate reduction of hematoma size. Furthermore, repeat scans might allow the identification of possible early predictors of successful cSDH volume reduction or resolution after 6 months.27
To monitor treatment effect and hematoma evolution across time, 2 more scans at 14–28 days (2–4 weeks) and 60–90 days (2–3 months) are also suggested.
Parameters of Radiographic Progression
It is unclear which radiographic parameter is the most relevant for the definition of progression; a postoperative increase in cSDH volume might not necessarily correspond to a progression in MLS or SDH width due to brain atrophy. However, a progression in MLS will more likely correspond to an increase in cSDH volume. In contrast, cSDH width might increase on the basis of hematoma organization and shape alterations, but the overall volume could remain the same. The application of different thresholds for each of these parameters to define progression also affects the sensitivity and specificity across these radiologic measures.
It is, therefore, vital that the reproducibility and comparability of longitudinal measurements be taken into account for each parameter. Volume changes corresponding to a few voxels that might have been segmented differently at the edge of the hematoma might already lead to an increase or decrease in volume. Here, automatic artificial intelligence–based segmentation algorithms are likely more accurate and could increase reproducibility, but further development and diagnostic accuracy studies are needed to confirm this possibility. Neural networks can be used to automatically segment cSDH to obtain more accurate volume measurements closer to the criterion standard. However, to our knowledge, only 1 study has applied this automatic segmentation technique specifically to patients with cSDH so far.28 In their study, Kellogg et al28 used a convolutional neural network to segment cSDH on CT scans, achieving an average DICE score of 0.806. However, this technique might be limited when it comes to the segmentation of isodense cSDH. and so far, there is no broader availability of this technique.
Furthermore, width measurements in longitudinal studies might be dependent on hematoma-shape changes and, therefore, are not directly comparable. However, any form of measurable recurrence might indicate a later progression with concurrent clinical symptoms. Therefore, radiographic parameters should always be evaluated and reported in the context of clinical information and, as much as possible, at similar time points with similar sets of related outcome measures.
Advantages and disadvantages of all parameters to indicate radiographic progression are shown in Table 2.
Advantages and disadvantages of singular and combinations of radiographic measurements to evaluate cSDH progression
Recurrence and Progression as Study End Points in cSDH Embolization Trials
Recurrence and progression are frequently included as either primary or secondary end points in the cSDH embolization trials.29 However, as shown in the Online Supplemental Data, there is no official standardized definition of radiographic hematoma recurrence or progression.
Furthermore, the application of thresholds and various definitions of primary and secondary radiologic end points also leads to altered sensitivities and specificities for the detection of recurrence and progression. Using radiographic progression as a study end point, therefore, poses some challenges, especially when considering the individual clinical relevance. However, due to the nature of the Onyx embolization agent (Covidien), we believe that a combination of both clinical and radiologic outcome measures is vital to the integrity of the trials and their ability to potentially change clinical practice. First, the addition of micronized tantalum powder to Onyx results in it being radiopaque.30 This means that a surgeon viewing the posttreatment CT or angiography scans cannot be blinded to the assignment of the patient to the control or treatment arm, which could lead to substantial bias with regard to further decision-making.
Furthermore, while clinical outcomes are generally of more value due to the above-provided reasons, no validated clinical measurement tool for cSDH exists, leaving room for imprecision and further bias. For example, it is difficult to say whether a patient with dementia who presented with mild headache and confusion and is now less confused following treatment constitutes a clinical improvement. This is in contrast to stroke trials, in which the National Institutes of Health Stroke Scale and modified Rankin Scale allow consistent, validated reporting of clinical outcomes. As a result, a trial that reports differing rates of recurrence requiring repeat surgery between the 2 arms on the basis of clinical assessment (a “soft” measure) would need to corroborate this outcome with concurrent radiographic evidence of cSDH volume reduction (a “hard” measure). It is critical, therefore, that the volumetric measurements are as accurate as possible. Due to the previously mentioned variations in hematoma size, morphology, density, and location, the only way to consistently achieve this accuracy is to perform section-by-section computer-assisted delineation of volumes followed by summation (ie, the criterion standard). The commonly applied ABC/2 methodology and its derivations are subject to too much variation and are, thus, insufficient as an outcome measure.
DISCUSSION
Recommendations for Standardizing Outcome Reporting
On the basis of these factors, we propose the standardized reporting of a clinical outcome (ie, rate of recurrence requiring surgery) and radiographic change as measured by manual computer-assisted volumetric analysis as outcome measures for the cSDH embolization trials. While such a measurement may seem cumbersome for the clinical routine, it provides the greatest potential to gain quality evidence. If the trial results should prove positive, adjustment for increased ease of implementation could be addressed at a later time point in conjunction with ongoing communication about the most clinically meaningful radiologic measures and best practices for using them (eg, manual versus automated) to maximize specificity, sensitivity, and replicability.
In addition, following completion of the trials, the standardization of the measurement technique, as well as the follow-up intervals, would facilitate meta-analysis of the results, boosting the quality of evidence for otherwise underrepresented subgroups, and provide more concrete guidance to the study design in future trials. Also, while there are likely to be differences in techniques and software when implementing a standard methodology, every center will have, for example, a standard axial CT scan of 5 mm. This would be particularly important if the trials were to report conflicting results or if results of a few are not positive. To this end, it will be critical to demonstrate that the radiographic outcome is in line with the clinical outcome, be it symptomatic improvement or reduction of necessary repeat interventions.
Because radiologic parameters seem to play a minor role when it comes to the definition of in- or exclusion criteria for clinical trials and because the selection of in- or exclusion criteria is very dependent on the scope and design of the trial, providing recommendations is outside the scope of this article. However, when radiologic criteria are applied, we suggest the same methods as we defined for radiologic outcome parameters and strongly encourage detailed documentation of methodology, while also emphasizing the need for the homogenization of measurement techniques.
Acceptable Variations
After initial training on the specific study standards, a constant quality control is required to guarantee a low interobserver variability, both for imaging core lab readers and for the core lab readers with the radiologists at the clinical sites. On the basis of our own unpublished observations, the deviation of MLS in individual patients among core lab readers and between local readings and the core lab should be <2 mm in at least 80% of the cases. The deviation of cSDH thickness measurements in individual patients should be <2 mm among core lab readers and <3 mm between local readings and those of the core lab (in at least 80% of the cases).
Limitations
Our common-data-elements review of existing cSDH trials has several limitations: First and most important, we based our analysis on reported protocols for collection of end points based on clinicaltrials.gov and other public-facing documentation of these studies and, therefore, may not have captured all study practices. However, this issue is yet another important part of study reporting—full and transparent outcome reporting from the stage of protocol drafting through to publication is necessary for replicable and transparent research. As we described throughout this article, there are very few published studies addressing the methodologic validity of imaging-based outcome measures specifically in cSDH. The here-proposed radiologic outcome parameters and measurement techniques are, therefore, based on current practice, practicability, and basic knowledge endorsed by the European Society of Minimally Invasive Neurological Therapy consortium. Furthermore, our recommendations reflect the research and experience of a limited group of experts, and further open dialogue is necessary to confirm the appropriateness of our recommended outcome set for cSDH trials. In addition, we would like to emphasize the need for further research addressing the reliability of measurement techniques defining radiologic outcome parameters in clinical trials of cSDH embolization. However, with this work, we aimed to highlight the need for homogenization and clear definition of outcome measures and hope to initiate further discussion and elucidate research concerning this topic.
CONCLUSIONS
Moving toward the standardization of radiologic outcome measures and measurement techniques in cSDH would increase the impact and significance of each embolization trial. Many open questions remain, especially with regard to the evaluation of the applicability and validity of radiologic outcome measures such as volume, width, and MLS in postoperative scans and as treatment-monitoring options. Currently, it seems as though manual computer-assisted measurements of cSDH volume represent the only viable option for sufficient accuracy. Furthermore, the definition of the optimal trial end point remains unclear and is highly dependent on the hypothesis to be tested. However, in order for the results of the currently running cSDH embolization trials to change clinical practice, we believe a combination of radiologic and clinical outcome measures is necessary. It is possible and reasonable that once the role of embolization for cSDH is well-established, a more simplified user-friendly version of hematoma measurement that has been adequately validated against the criterion standard of manual computer-assisted volume measurements may be used in day-to-day practice. However, the major findings here—that radiologic outcome reporting in currently active cSDH trials is highly heterogeneous and noncomparable—require open, rapid, and ongoing communication about study design among neurointerventionalists to ensure that these trials contribute to larger-scale, comparable outcomes research in support of evidence-based practice.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received November 23, 2021.
- Accepted after revision February 8, 2022.
- © 2022 by American Journal of Neuroradiology