Abstract
BACKGROUND AND PURPOSE: The association between hereditary hemorrhagic telangiectasia and intracranial aneurysms remains controversial. This study evaluated the prevalence and characteristics of intracranial aneurysms in patients with hereditary hemorrhagic telangiectasia with brain vascular malformations.
MATERIALS AND METHODS: Between 2007 and 2021, patients enrolled in the Brain Vascular Malformation Consortium with definite hereditary hemorrhagic telangiectasia, the presence of brain vascular malformations, and available angiographic studies of the brain were retrospectively reviewed. Angiographic features of intracranial aneurysms and their relationship to coexisting brain vascular malformations were analyzed. We also examined the association between baseline clinical features and the presence of intracranial aneurysms.
RESULTS: One hundred eighty patients were included. A total of 14 intracranial aneurysms were found in 9 (5%) patients, and 4 intracranial aneurysms were considered flow-related aneurysms. Patients with intracranial aneurysms were significantly older than patients without intracranial aneurysms (mean, 48.1 [SD, 18.2] years versus 33.5 [SD, 21.0] years; P = .042). If we excluded flow-related intracranial aneurysms, the prevalence of intracranial aneurysms was 3.3%. All intracranial aneurysms were in the anterior circulation, were unruptured, and had an average maximal diameter of 3.9 (SD, 1.5) mm. No intracranial aneurysms were found in pediatric patients with hereditary hemorrhagic telangiectasia. No statistically significant correlation was observed among other baseline demographics, hereditary hemorrhagic telangiectasia features, and the presence of intracranial aneurysms.
CONCLUSIONS: The prevalence of intracranial aneurysms in this large cohort study is comparable with that in the general population and might be increased slightly due to hemodynamic factors associated with shunting brain vascular malformations.
ABBREVIATIONS:
- HHT
- hereditary hemorrhagic telangiectasia
- IA
- intracranial aneurysm
- VM
- vascular malformation
Hereditary hemorrhagic telangiectasia (HHT) or Osler-Weber-Rendu syndrome is an autosomal dominant disorder characterized by mucocutaneous telangiectasias and vascular malformations (VMs) in organs, including the liver, gastrointestinal tract, lungs, brain, and spinal cord.1,2 It is caused by mutations in one of several genes involved in the transforming growth factor-β/bone morphogenetic protein signaling pathway. Loss-of-function mutations in HHT genes have been shown to impair endothelial cell proliferation and migration during angiogenesis and vascular remodeling.1,3,4 Approximately 96% of individuals with a definite clinical diagnosis of HHT have a mutation in the endoglin (ENG) gene or activin A receptor type II-like kinase 1 (ACVRL1) gene,5 and about 3% of patients have a mutation in the SMAD4 gene. The diagnosis of HHT is made clinically using the Curaçao criteria, which include spontaneous and recurrent nose bleeds, multiple mucocutaneous telangiectasias (of the lips, oral cavity, nose, or fingers), visceral VMs (of the liver, lungs, or CNS), and a first-degree relative with HHT. Patients are diagnosed with “definite HHT” when 3 of these 4 criteria are met, whereas those who fulfill 2 criteria are considered to have “possible HHT.”6 For asymptomatic individuals from a family with known HHT, the International HHT Guidelines recommend genetic testing for diagnosis.5
Patients with HHT present with a wide variety of cerebrovascular diseases. Between 10% and 20% of patients have brain VMs, classified in previous studies as pial AVFs, nidus-type AVMs, or capillary vascular malformations.7⇓⇓⇓-11 Other nonshunting vascular lesions such as cavernous malformations, developmental venous anomalies, and intracranial aneurysms (IAs) were also found in patients with HHT.8,11,12
Although earlier studies reported the prevalence of IAs in the HHT population,8,11,13,14 no studies have addressed the characteristics of these aneurysms and their relationship to AVMs. The present study aimed to determine the prevalence of IAs in the HHT population, identify angiographic features of IAs, and examine the correlation between IAs and brain VMs in patients with HHT. Notably, a recent study found a strikingly high 14.5% prevalence of IAs in an HHT cohort, contrary to previous studies.14 Current screening recommendations may need refinement if this higher prevalence of IAs is confirmed in our data set.
MATERIALS AND METHODS
Study Population
We analyzed patient data from the HHT project of the Brain Vascular Malformation Consortium. Patients with either a confirmed genetic diagnosis or a definite clinical diagnosis of HHT, based on the presence of at least 3 of the Curaçao criteria,6 were enrolled in the Brain Vascular Malformation Consortium HHT project as previously reported.10,15 The Brain Vascular Malformation Consortium HHT cohort aimed for a 25% recruitment target for patients with brain VMs.15 From January 2007 to December 2021, we included all recruited patients with brain VMs and available angiographic studies (ie, CTA, MRA, and/or DSA). All patients provided written, informed consent. The study was approved by each participating center's institutional review board.
Data Collection
For each patient, we collected data including age at enrollment, sex, clinical presentations, family history, and genetic testing results (ENG, ACVRL1, SMAD4) when available. Radiologic data included biplanar 6-vessel selective cerebral angiography, contrast-enhanced MRA or TOF-MRA using 1.5T or 3T scanners, and CTA. All images were retrospectively reviewed by 2 of the authors (H.-C.C. and T.K.) in consensus. Images were assessed for the presence of IAs and shunting lesions such as AVMs and AVFs, as classified in previous studies.10,16 The aneurysm location, the number of aneurysms per patient, their geometric measurements, and their relationship to the intracranial shunting lesions were recorded. We measured aneurysm neck width, dome-to-neck ratio, and aspect ratio on the PACS. Aneurysms with a neck diameter of ≥4 mm or a dome-to-neck ratio of <2 were classified as wide-neck.17,18 A Spetzler-Martin grade was calculated for each AVM when the patient had a concurrent IA.19 IAs were considered unrelated aneurysms when they presented on vessels that were not AVM feeders, whereas those arising from vessels contributing to perfusion of the nidus were termed flow-related aneurysms.20 Flow-related aneurysms were further classified as proximal when located at the circle of Willis up to the primary bifurcation, while those more distally located were considered distal.
Statistical Tests
We determined the prevalence of IAs by the number of patients having at least 1 IA divided by the total number of included patients with HHT. We tested for differences in age, sex, the presence of Curaçao criteria, and the presence of AVMs/AVFs between patients with IAs and those without. Continuous variables were compared using the Student t test, and categoric variables were compared using the Fisher exact test. We considered P values < .05 as statistically significant. All analyses used SPSS (IBM).
RESULTS
One hundred eighty patients with HHT and brain VMs as well as available angiographic studies were included in the study. The average age at enrollment was 34.2 (SD, 21.1) years, with 54 (30%) subjects younger than 18 years of age. Female patients accounted for 61.7% of the population. One hundred thirty-one patients had positive findings on genetic testing: One hundred nine (83.2%) had an ENG mutation, 21 (16.0%) had an ACVRL1 mutation, and 1 (0.7%) had a SMAD4 mutation. Basic demographics and clinical features of the study population are summarized in Table 1.
Basic demographics of 180 subjects with HHT and brain VMs
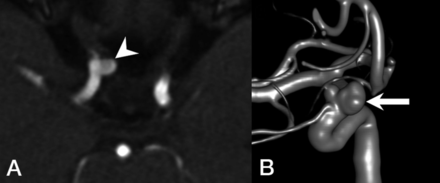
Fourteen IAs were noted in 9 (5%) patients, and 3 (33%) patients had multiple aneurysms. No pediatric patients with HHT in this cohort had an IA. All IAs were unruptured and were found in the anterior circulation, most commonly located in the ICA (64%), followed by the anterior cerebral artery (21%). The average neck size and maximal aneurysm diameter were 3.7 (SD, 1.3) mm and 3.9 (SD, 1.5) mm, respectively. The average aspect ratio was 0.92 (SD, 0.30). All aneurysms had a dome-to-neck ratio of <2. A representative example of IAs is shown in Fig 1.
Representative images of a patient with HHT with an IA unrelated to AVMs. A, MRA demonstrates an ophthalmic segment aneurysm of the right ICA (arrowhead) projecting medially. B, 3D reconstruction of a DSA shows a bilobed, wide-neck, right ophthalmic segment aneurysm of the right ICA (arrow).
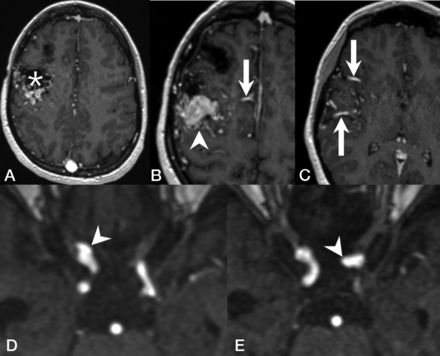
Of the 9 patients with IAs, 6 had nidus-type brain AVMs, 1 had an AVF, and 2 had a capillary vascular malformation. Ten (71%) of the 14 IAs were considered unrelated to the VMs, whereas 4 (29%) were considered flow-related aneurysms arising from a feeding artery of a shunting VM. One patient with a right frontal Spetzler-Martin grade III AVM and a nidus size of 4 cm had 1 aneurysm on each of the ICAs (Fig 2). Another patient with a left frontal AVF and a large venous pouch had an aneurysm on the ipsilateral anterior cerebral artery. The last patient had 2 Spetzler-Martin grade I AVMs in the same hemisphere as the aneurysm. The characteristics of IAs and angiographic features are summarized in the Online Supplemental Data. Patients with IAs were significantly older compared with patients without IAs (mean, 48.1 [SD, 18.2] years versus 33.5 1 [SD, 21.0] years; P = .042). No statistically significant association was found between patients with or without IAs regarding sex, the presence of the Curaçao criteria, or the presence of concurrent shunting brain VMs (AVMs and/or AVFs) (Table 2).
Representative images of a patient with HHT with flow-related cerebral aneurysms. T1 contrast-enhanced MR images demonstrate a Spetzler-Martin grade III right frontal AVM with a nidus size of 4 cm (A, asterisk) and venous varices (B, arrowhead), supplied by several dilated arterial feeders from the right anterior cerebral arteries (B, arrow) and the right middle cerebral arteries (C, arrow). MRA demonstrates a clinoid segment aneurysm of the right ICA (D, arrowhead) and another aneurysm arising from the ophthalmic segment of the left ICA (E, arrowhead).
Bivariate analysis between baseline demographics and IAs
DISCUSSION
The association between HHT and IAs remains controversial. In a report on neurovascular manifestations of patients with HHT, Brinjikji et al11 demonstrated a 2.1%. prevalence of IAs. Woodall et al8 evaluated 230 patients with HHT and found a 2.4% prevalence of IAs. Ring et al13 looked for arterial aneurysms in all locations in an HHT cohort and reported a 4.3% IA prevalence. A very recent study from Perez Akly et al14 found 33 (14.5%) patients with HHTs with IAs, >3-fold higher than the prevalence rate of 3.2% in the general population,21 thus potentially changing the clinical practice regarding active screening for IAs in patients with HHT. This remarkable discrepancy and the potential impact of encountering a high prevalence of IAs in patients with HHT prompted the present study.
We report a prevalence of IAs of 5% in this largest series of patients with HHT with brain VMs. In the subgroup of patients not having flow-related IAs, the prevalence of IAs was 3.3%, very similar to that of the general population. Although we included specifically patients with HHT with brain VMs, our observation is in line with the reported prevalences by Brinjikji et al,11 Woodall et al,8 and Ring et al.13 In addition, our study cohort shares similar prevalences of epistaxis,8,11,14 mucosal telangiectasias,1,11,22 and pulmonary AVMs1,11,23,24 with previous reports, and, therefore, is likely generalizable to unselected patients with HHT. Because children can develop complications of HHT,25 we believe that it is necessary to include the pediatric population in this epidemiologic study to better understand the prevalence and natural history of IAs and, more important, to determine whether HHT is associated with an increased risk of IAs. Moreover, we are convinced that by including all patients with available angiographic studies of all types (ie, CTA, MRA, and/or DSA), our observed prevalence of IAs more accurately reflects the prevalence of IAs in the HHT population. According to our findings and those of the latter 3 studies,8,11,13 the prevalence of IAs in HHT is similar to that in the general population, with a small additional prevalence of IAs in patients with HHT with coexisting shunting brain VMs.
This observation is in keeping with pathogenetic concepts of HHT in several ways. First, it is postulated that in HHT, the targets of the dysfunction are the venules instead of the arteries.16 Early histopathologic studies demonstrated that the clinically detectable lesions of HHT start with focal dilation of postcapillary venules, which enlarge and connect with arterioles, eventually forming direct arteriovenous shuntings as capillaries disappear.1,26 More recent animal studies also suggest that the ENG/ACVRL1 signaling pathway mediates the endothelial cell shear stress response and is essential for flow-induced endothelial cell migration against the direction of blood flow, starting from growing veins, ie, flow-migration coupling. Loss of ENG or ACVRL1 expression in venous and capillary endothelial cells is sufficient to cause AVMs as a result of impaired flow-migration coupling, whereas ENG or ACVRL1 deletion in arterial endothelial cells does not lead to AVM formation.4,27⇓⇓-30 Histopathologically, HHT is unlikely to be associated with a higher prevalence of IAs. Second, although several reports have associated the interruption of the transforming growth factor-β signaling pathway with a higher prevalence of visceral aneurysms in patients with HHT due to its important role in extracellular matrix remodeling,13,31 there is no genetic evidence to support applying the same hypothesis to IAs. ENG, one of the most commonly found mutated genes in patients with HHT, has repeatedly been reported to have no association with IAs.32,33
In a study about sequencing transforming growth factor-β pathway genes in familial IA cases, Santiago-Sim et al34 demonstrated that mutations in transforming growth factor-β receptor genes are not a major cause of IAs. Furthermore, a recently published genome-wide association study also demonstrated no evidence of polygenic overlap between other aortic aneurysms and IAs,35 while another meta-analysis of genome-wide association study on genetic risk factors for IAs found no linkage between transforming growth factor-β-related genes and the development of IAs.36 Finally, unlike some other clinical features of HHT that become apparent in early childhood and evolve, no IAs were found in the pediatric population of our cohort. In addition, the mean age of patients with IAs in this study was 48 years, comparable with the mean age of 47–55 years described in non-HHT populations with IAs.37,38 If IAs and VMs share the same pathogenesis, there would presumably be more evidence of IAs in younger patients with HHT.
IAs in this study were most commonly found in the ICA and were small with a mean maximal diameter of 3.9 mm. Similar to findings in the previous studies,8,11,14 no patients in this cohort presented with SAH, which may hint at a benign nature of IAs in HHT. Although there were a few case reports describing ruptured IAs in patients with HHT,39,40 most were flow-related aneurysms associated with a shunting brain VM.
In this study, we found no association between IAs and shunting brain VMs. Because approximately 71% of included patients with HHT had at least 1 shunting brain VM, it is possible that we could not detect any significant difference in brain VM prevalence between patients with and without IAs, given this high prevalence of brain VMs in our cohort. Notably, merely 29% of the IAs in this study were related to the shunting brain VMs, and only 2% of the AVMs were found to have flow-related aneurysms, as opposed to 18% described previously by a meta-analysis on the natural history of brain AVMs and concurrent IAs in the non-HHT population.41 One possible explanation is thart most of the AVMs found in patients with HHT are <3 cm;10,11,42 therefore, the hemodynamic factors related to the shunting in the AVM nidus, such as higher shear stress on the vessel wall, are less prominent among patients with HHT.
In conclusion, the prevalence of IAs in patients with HHT with brain VMs is similar to that in the general population. In addition, we observed that IAs in patients with HHTs are seen in adults, but not children, and they are typically small with a low likelihood of hemorrhagic presentation. Thus, our observations do not support a recommendation for routine screening of patients with HHT specifically for IAs.
This study has limitations. First, it has some inherent selection bias because we included only patients with brain VMs. Although the genetic linkage between IAs and brain VMs, if any, remains undetermined, the prevalence of IAs in our study was in line with that reported in the literature. Second, genetic information was not available for one-third of the included individuals; therefore, there was an insufficient sample size to assess the association between IAs and genes mutated. Also, our study cohort included few patients with ACVRL1 or SMAD4 mutations, unsurprisingly, given the known association between brain VMs and ENG mutation, among patients with HHT. Additional studies will be required to clarify the association between these genes and IAs. Finally, the limited sample size of IAs may reduce the power of the study to detect statistically significant correlations such as the association between IAs and shunting brain VMs, but there was no evidence of even a trend association.
CONCLUSIONS
From this large cohort of patients with HHT and brain VMs, we demonstrate that the prevalence of IAs in HHT is similar to that of the general population and is not clearly associated with the presence of shunting brain VMs. Further studies are required to explore the mechanistic linkage between HHT and IA formation and the natural history of IAs in the HHT population.
ACKNOWLEDGMENTS
We would like to thank Professor Shiao-Chi Wu of National Yang Ming Chiao Tung University for statistical analysis.
Brain Vascular Malformation Consortium Hereditary Hemorrhagic Telangiectasia Investigator Group:
Mary E. Atherton, Murali M. Chakinala, Marianne S. Clancy, Marie E. Faughnan, James R. Gossage, Adrienne M. Hammill, Katharine Henderson, Steven Hetts, Peter Hountras, Vivek Iyer, Raj S. Kasthuri, Helen Kim, Timo Krings, Michael T. Lawton, Doris Lin, Johannes Jurgen Mager, Douglas A. Marchuk, Justin P. McWilliams, Jamie McDonald, Ludmila Pawlikowska, Jeffrey Pollak, Felix Ratjen, Karen Swanson, Dilini Vethanayagam, Shantel Weinsheimer, Andrew J White, Pearce Wilcox.
Footnotes
The Brain Vascular Malformation Consortium is part of the Rare Diseases Clinical Research Network, which is funded by the National Institutes of Health and led by the National Center for Advancing Translational Sciences through its Office of Rare Diseases Research. The Brain Vascular Malformation Consortium is funded under grant No. U54NS065705 as a collaboration between the National Center for Advancing Translational Sciences and the National Institute of Neurological Disorders and Stroke. All Rare Diseases Clinical Research Network consortia are supported by the network's Data Management and Coordinating Center (U2CTR002818). Funding support for the Data Management and Coordinating Center is provided by National Center for Advancing Translational Sciences and the National Institute of Neurological Disorders and Stroke.
M.E.F. was also supported by the Li Ka Shing Knowledge Institute. This work was funded, in part, by the Holt-Hornsby and Andreae Fund and University Medical Imaging Toronto.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received July 24, 2023.
- Accepted after revision October 6, 2023.
- © 2023 by American Journal of Neuroradiology