Abstract
BACKGROUND AND PURPOSE: Currently, there is no effective treatment for pediatric patients with complete spinal cord injury. Motor imagery has been proposed as an alternative to physical training for patients who are unable to move voluntarily. Our aim was to reveal the potential mechanism of motor imagery in the rehabilitation of pediatric complete spinal cord injury.
MATERIALS AND METHODS: Twenty-six pediatric patients with complete spinal cord injury and 26 age- and sex-matched healthy children as healthy controls were recruited. All participants underwent the motor imagery task-related fMRI scans, and additional motor execution scans were performed only on healthy controls. First, we compared the brain-activation patterns between motor imagery and motor execution in healthy controls. Then, we compared the brain activation of motor imagery between the 2 groups and compared the brain activation of motor imagery in pediatric patients with complete spinal cord injury and that of motor execution in healthy controls.
RESULTS: In healthy controls, compared with motor execution, motor imagery showed increased activation in the left inferior parietal lobule and decreased activation in the left supplementary motor area, paracentral lobule, middle cingulate cortex, and right insula. In addition, our results revealed that the 2 groups both activated the bilateral supplementary motor area, middle cingulate cortex and left inferior parietal lobule, and supramarginal gyrus during motor imagery. Compared with healthy controls, higher activation in the bilateral paracentral lobule, supplementary motor area, putamen, and cerebellar lobules III–V was detected in pediatric complete spinal cord injury during motor imagery, and the activation of these regions was even higher than that of healthy controls during motor execution.
CONCLUSIONS: Our study demonstrated that part of the motor imagery network was functionally preserved in pediatric complete spinal cord injury and could be activated through motor imagery. In addition, higher-level activation in sensorimotor-related regions was also found in pediatric complete spinal cord injury during motor imagery. Our findings may provide a theoretic basis for the application of motor imagery training in pediatric complete spinal cord injury.
ABBREVIATIONS:
- CSCI
- complete spinal cord injury
- FWE
- family-wise error
- HC
- healthy control
- KVIQ-10
- Kinesthetic and Visual Imagery Questionnaire
- IPL
- inferior parietal lobule
- ME
- motor execution
- MI
- motor imagery
- PCL
- paracentral lobule
- SMA
- supplementary motor area
Traumatic spinal cord injury is a sudden and unpredictable incident that can destroy the spinal cord, leading to sensory and motor dysfunction.1 It can occur at any age, including childhood, not only causing a heavy burden on the families but also having serious consequences for children’s physical and mental well-being.2 The most common rehabilitation strategy used in patients with incomplete spinal cord injury is physical training, which is intended to promote cortical plasticity by driving motor neural networks and facilitating functionally relevant muscle activity below the injury level.3 However, due to the complete disruption of afferent and efferent pathways,4 pediatric patients with complete spinal cord injury (CSCI) are not able to execute physical movement. At present, there is no effective treatment for pediatric CSCI. Currently, motor imagery (MI) is being studied as a potential treatment for the functional recovery of motor dysfunction diseases5,6 because it can activate sensorimotor networks and can be performed by injured patients who are unable to move voluntarily.7⇓-9
MI is the mental simulation of a movement in the absence of obvious motor output.10 The link between MI and motor execution (ME) has been empirically demonstrated in adult studies.11,12 A number of neuroimaging studies have repeatedly revealed overlapping neural activity during MI and ME,12 including the premotor cortices, supplementary motor area (SMA), basal ganglia, and cerebellum.10 MI may reinforce these crucial motor networks and improve the motor skills of healthy individuals, including athletes13 and musicians.14 Additionally, MI may be an alternative to physical training in cases where movement is severely impaired or not possible, such as when patients have CNS injuries including stroke,7,8 Parkinson’s syndrome,9 MS,15 and spinal cord injury.16
Compared with adult patients, children have a greater possibility of developing neuroplasticity because their brains are undergoing rapid growth and development;17 therefore, effective therapies may lead to satisfactory rehabilitation in children.18 According to previous studies,19,20 children 5–6 years of age have already demonstrated MI abilities, and it has been reported that MI training can also improve the motor function of children with cerebral palsy,18,21 developmental coordination disorder,22,23 and perinatal stroke.5 The application of MI training in the motor rehabilitation of adults with CNS injury and children with cerebral palsy, developmental coordination disorder, or perinatal stroke suggests that it may also be helpful for the rehabilitation of pediatric patients with CSCI. However, there is no research on the application of MI in pediatric patients with CSCI, and the neural mechanism of MI in the rehabilitation of pediatric patients with CSCI is unclear.
In this study, we intended to apply task-based fMRI to study the alterations in brain activation in pediatric patients with CSCI during MI and to reveal its potential mechanisms for motor rehabilitation, which may provide insights into the potential applications of MI training in pediatric patients with CSCI.
MATERIALS AND METHODS
Participants
After obtaining approval from the Medical Research Ethics Committee of Xuanwu Hospital, all participants were enrolled, and informed consent was obtained from the parents or guardians of the children. On admission to the hospital, all patients were evaluated by 2 experienced physicians, using the Glasgow Coma Scale, Posttraumatic Amnesia Scale, and loss of consciousness assessment. Then, the American Spinal Injury Association scale was administered to evaluate the sensory and motor functions of the pediatric patients with CSCI. After that, the Kinesthetic and Visual Imagery Questionnaire (KVIQ-10)24 was conducted to evaluate the MI abilities of all participants. To be included in this study, all patients had to meet following requirements: 1) history of traumatic CSCI; 2) between 6 and 12 years of age; 3) time since injury ≥2 months; 4) KVIQ-10 score ≥25;25 5) no associated traumatic brain injury confirmed by conventional MR imaging; 6) no history of mental disorder, mental illness, epilepsy, or cognitive disorder; and 7) no contraindication for MR imaging. The inclusion criteria of healthy controls (HCs) were as follows: 1) age, sex, and education matched those of pediatric patients with CSCI; 2) KVIQ-10 score ≥ 25; 3) no history of mental disorder, mental illness, epilepsy, or cognitive disorder; and 4) no contraindication to MR imaging.
Experimental Tasks
In this study, the HCs underwent both MI and ME task-based fMRI scans, and the pediatric patients with CSCI underwent only the MI task. HCs performed the ME task with right-ankle dorsiflexion, and all participants performed the MI task using a kinesthetic MI of right-ankle dorsiflexion. During the experiment, each participant conducted a prescan practice, underwent task-based fMRI scans, and was asked to complete a questionnaire after the fMRI scans. All processes were completed with the aid of the parents or guardians of the recruited children.
During the prescan practice, all participants familiarized themselves with the procedure of the tasks to ensure proper execution. Our rehabilitation physicians conducted the intensive ME simulation training for all HCs. ME training used a recording of ME produced by our rehabilitation medicine department. In the beginning, the HCs relaxed for 3 minutes, then a rehabilitation physician explained and demonstrated the ME of right-ankle dorsiflexion, and the HCs followed. Then following the guidance of the ME recording, all HCs conducted the ME of ankle dorsiflexion step by step. MI training used an MI therapy recording produced by our rehabilitation medicine department. First, the participants relaxed for 3 minutes, then a rehabilitation physician explained and demonstrated the MI of the right ankle dorsiflexion, and the participants followed. To ensure that all participants could understand and accurately perform the MI of ankle dorsiflexion, the participants repeated this process 3 times. After that, following the guidance of the MI recording, participants performed the MI of ankle dorsiflexion step-by-step. All processes were conducted in a quiet environment.
In the design of the task experiment, for the first 10 scans, no task was given so that participants could adjust and adapt themselves. Then, 4 repetitions were completed, which alternated between 10 scan task blocks and 10 scan rest blocks (task block first, rest block last). The ME task required HCs to conduct active ankle dorsiflexion (from dorsiflexion 20° to plantarflexion 30°) using their own pace (an approximate frequency of 0.5–1.0 Hz). A small pillow was placed under each participant’s popliteal fossa to make ankle movement easier. While the patient performed the tasks, foam pads were used to minimize head motion. In MI tasks, both HCs and pediatric patients with CSCI were asked to imagine themselves performing the same movements as the right ankle dorsiflexion, rather than actually performing them. For the MI tasks to be performed successfully, an operator must visually observe the entire task procedure to ensure that no overt movement occurred during either the MI or rest blocks.
As reported in questionnaire interviews after the fMRI scan, all participants in this study were able to complete the MI tasks, and they did not perform any imagery during the rest blocks.
MRI.
Imaging scans were acquired on a 3T MR imaging scanner (Siemens) using 12-channel phased array head coils. To avoid visual input, participants were asked to lie supine and close their eyes. The conventional brain axial FLAIR sequence was acquired first to rule out cerebral abnormalities. The images were collected with a single-shot gradient-echo-planar imaging sequence. The acquisition parameters for the scans were as follows: Each volume consisted of 35 axial slices (section thickness = 3 mm and interslice gap = 1 mm), TR = 2000 ms, TE = 30 ms, flip angle = 90°, matrix = 64 × 64, field of view = 220 × 220 mm2, readout bandwidth = 2004 Hz/px, and final voxel size = 3.4 × 3.4 × 4.2 mm3.
Data Preprocessing
Data preprocessing was performed using Statistical Parametric Mapping, Version 12 (SPM12; http://www.fil.ion.ucl.ac.uk/spm) based on Matlab 2013b (MathWorks). The first 10 time point images were discarded to ensure stabilization of the signal. Then, the remaining images were slice-timing-corrected using the middle slice as a reference, and all volumes were spatially realigned to correct for head movements. Realignment of the volumes was followed by checking the head-movement parameters, and the data sets with more than 3 mm maximum translation or 3° maximum rotation were discarded. For spatial normalization of the realigned volumes, the data were then spatially normalized to a pediatric brain template from the 6- to 12-year age group in Montreal Neurological Institute space (https://www.nitrc.org/projects/chn-pd). Then, the images were resampled to 3 × 3 × 3 mm3 voxel size. In the final step, a Gaussian kernel with an 8-mm full width at half maximum was applied to smooth the data.
Statistical Analysis
The statistical analyses were performed with SPM 12 implemented in Matlab 2013b. In the first level of analyses, brain activations during tasks were estimated using a general linear model. The blood oxygen level–dependent signal time courses were convolved with a canonical hemodynamic response function. A 6-parameter rigid body transformation (3 translations, 3 rotations) derived from the head-motion correction was added in the general linear model as covariates of no interest. A standard high-pass filter (128-second cutoff) was used to eliminate low-frequency signal drift. After the model estimation, by comparing experimental conditions with respective control conditions, the contrast within each task was defined to obtain task-related activations. Second-level analyses were then performed using the contrast images.
In the second-level group analyses, one-sample t tests were conducted to identify brain regions significantly activated by MI in both groups or ME in HCs. Statistical significance was determined on the basis of cluster-level family-wise error (FWE) correction (P < .05, 2-tailed). A paired t test was used for the comparison of brain activation during MI and ME tasks in HCs (cluster-level FWE correction with P < .05). With age and sex as nuisance covariates, two-sample t tests were conducted to compare the activation of the brain between pediatric patients with CSCI and HCs during MI and the activation of the brain between pediatric patients with CSCI during the MI task and HCs during the ME task (cluster-level FWE correction with P < .05).
Spearman analyses were performed to explore any possible correlation between the activation intensity of the regions with significant group differences during MI and the clinical variables of pediatric patients with CSCI (including injury duration, sensory scores, motor scores, and KVIQ-10 scores).
RESULTS
Demographic Information
Twenty-six pediatric patients with CSCI (23 girls and 3 boys, with a mean age of 8.04 [SD, 1.89] years and a range from 6 to 12 years) and 26 HCs (23 girls and 3 boys with a mean age of 8.65 [SD, 1.96] years and a range from 6 to 12 years) were enrolled in this study. Pediatric patients with CSCI had the disease for a duration ranging from 2 to 108 months, with a mean of 24.73 (SD, 24.15) months. All patients were classified as grade A according to the American Spinal Injury Association Impairment Scale 2012 (https://www.physio-pedia.com/American_Spinal_Cord_Injury_Association_(ASIA)_Impairment_Scale). The detailed information of pediatric patients with CSCI was provided by Online Supplemental Data. No differences were found in age (Mann-Whitney U test, P = .23) or sex (χ2, P = 1.00) between pediatric patients with CSCI and HCs.
Brain Activation
Brain Activation Patterns of HCs during ME and MI.
Figure 1A, -B shows significant activation of HCs during ME and MI. The ME and MI tasks of HCs evoked several similar brain regions, mainly including the bilateral SMA and left middle cingulate cortex.
Brain activation of HCs during ME and MI and the activation of pediatric patients with CSCI during MI. Typical brain regions with activation in HCs during ME are shown in A, mainly including the left PCL, PSMC, middle cingulate cortex, precuneus, and bilateral insula, SMA (uncorrected voxelwise P < .001, FWE-corrected cluster-level P < .05 and cluster ≥172). Typical brain regions with activation in HCs during MI in B, mainly including the left IPL and the bilateral SMA, middle cingulate cortex (uncorrected voxelwise P < .001, FWE-corrected cluster-level P < .05 and cluster ≥248). Typical brain regions with activation in pediatric patients with CSCI during MI are shown in C, mainly including the bilateral SMA, PCL, PSMC, putamen, pallidum, insula, superior temporal gyrus, middle cingulate cortex, cerebellar lobules III–V, and the left IPL, precuneus, and supramarginal gyrus (uncorrected voxelwise P < .001, FWE-corrected cluster-level P < .05 and cluster ≥199). PSMC indicates primary sensory and motor cortex.
In a direct comparison of maps between the ME and MI of HCs, the MI task showed significantly increased activation in the left inferior parietal lobule (IPL) and significantly decreased activation in the left SMA, paracentral lobule (PCL), middle cingulate cortex, and right insula (Fig 2 and Table 1).
Brain regions with significant differences between MI and ME in HCs. Compared with ME, MI of HCs shows increased activation in the left IPL (uncorrected voxelwise P < .001, FWE-corrected cluster-level P < .05 and cluster ≥113, red) as well as decreased activation in the left SMA, PCL, middle cingulate cortex, and right insula (uncorrected voxelwise P < .001, FWE-corrected cluster-level P < .05 and cluster ≥24; blue). R indicates right; L, left.
Brain regions with significant differences between MI and ME in HCs
Comparison of MI-Evoked Brain Activation Patterns between Pediatric Patients with CSCI and HCs
During the MI task, pediatric patients with CSCI and HCs showed similar activated brain areas, mainly including the bilateral SMA, middle cingulate cortex and left IPL, supramarginal gyrus (Fig 1B, -C). However, compared with HCs, pediatric patients with CSCI showed significantly increased activation in the bilateral PCL, SMA, putamen, and cerebellar lobules III–V during MI (Fig 3 and Table 2).
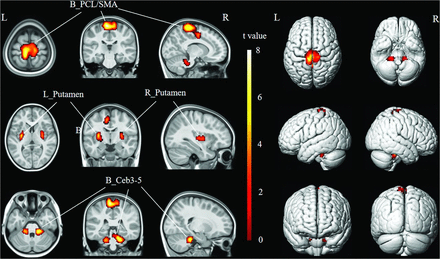
Brain regions with increased activation in pediatric patients with CSCI during MI. Compared with HCs, pediatric patients with CSCI show increased activation in bilateral PCL, SMA, putamen, and cerebellar lobules III–V (uncorrected voxelwise P < .001, FWE-corrected cluster-level P < .05 and cluster ≥91). R indicates right; L, left; Ceb3–5, cerebellar lobules III–V.
Brain regions with increased activation in pediatric patients with CSCI during MI
Comparison between MI-Evoked Brain Activation Patterns in Pediatric Patients with CSCI and ME-Evoked Brain Activation Patterns in HCs
Additionally, we observed that the MI task in pediatric patients with CSCI activated brain structures similar to those in the ME task in HCs. In comparison with the ME task in HCs, the MI task in pediatric patients with CSCI showed significantly increased activation in the right PCL, bilateral putamen, and left cerebellar lobules III–V (Online Supplemental Data).
Correlation Analyses between the Brain Activation and Clinical Variables in Pediatric Patients with CSCI
According to the Spearman correlation analyses, there were no correlations between the activation of brain regions with significant group differences during MI and the clinical variables (including injury duration, sensory scores, motor scores, and KVIQ-10 scores) in pediatric patients with CSCI (P > .05).
DISCUSSION
The present study explores the potential neural mechanism of MI in the rehabilitation of pediatric patients with CSCI. Our study included 2 major findings: First, motor-related areas (the bilateral SMA and left middle cingulate cortex) and cognitive-related regions (the left IPL and supramarginal gyrus) were functionally preserved in the MI of pediatric patients with CSCI. Second, the MI of pediatric patients with CSCI evoked higher activation in sensorimotor-related regions (bilateral PCL, SMA, putamen, and cerebellar lobules III–V) than that of HCs, and the activation of some of these regions that of pediatric patients with CSCI during MI was even higher than that of HCs during ME.
MI and ME in Healthy Children
Our findings showed that MI shares some neural substrates with ME in healthy children, including the bilateral SMA and left middle cingulate cortex, consistent with findings in previous studies in adults.12 MI may improve the motor skills of healthy children by activating brain areas shared with ME.26,27
In addition, compared with ME, MI in healthy children showed reduced activation in the left SMA, PCL, middle cingulate cortex, and right insula, which are thought to be involved in motor preparation, execution, and modulation.12 MI is an internal representation of movement in the absence of any obvious motor output,10 which may be responsible for the reduced activation of motor-related brain areas. Conversely, compared with ME, MI in healthy children showed increased activation in the left IPL, which plays a key role in higher cognitive functions.10,28 As proposed in a recent motor-cognitive model of MI, action simulation is more dependent on consciously controlled cognitive processes.26,29 This model can be further supported by the increased activation of the IPL in our results.
Brain Preservation and Reorganization during the MI Task in Pediatric Patients with CSCI
When performing MI, both groups activated not only motor-related areas (the bilateral SMA and middle cingulate cortex)12 but also cognitive-related regions (the left IPL and supramarginal gyrus).11 Our results revealed that many features of MI-evoked brain activation in pediatric patients with CSCI were consistent with those observed in healthy children. After CSCI, the functions of these brain regions may be preserved and can be activated by MI,10 possibly aiding pediatric patients in improving their sensory and motor functions, just as MI can assist healthy children in improving their motor skills.27
Additionally, during the MI task, pediatric patients with CSCI showed higher-level activation in a sensorimotor network that included the bilateral PCL, SMA, putamen, and cerebellar lobules III–V than HCs, and the activation in some of these brain regions in pediatric patients with CSCI during MI was even higher than that of HCs during ME. According to a previous study, task-related activation of the motor cortex is closely associated with motor recovery after subcortical stroke,30 indicating that overactivation of these regions is likely to reflect neural reorganization.11
The PCL, SMA, and putamen are all located in the corticostriatal sensorimotor circuit, which is a pathway from the motor areas of the frontal cortex (including the primary motor cortex, SMA, pre-SMA, and premotor cortex) to the putamen located in the striatum.31 The corticostriatal sensorimotor circuit is involved in motor control via glutamatergic projections.32 As part of the primary motor cortex, the PCL is not only involved in the cortical control of micturition and defecation but also responsible for the motor and sensory innervations of the lower extremities.33 The SMA is a cortical region located in the premotor cortex that plays important roles in the planning, preparation, and initiation of motor acts.34 At the level of the striatum, the sensorimotor circuit is largely centered on the putamen, which receives projections from the motor and sensory cortices,35 including the PCL and SMA. The putamen has been reported to be involved in reinforcement learning as well as motor control,36 and its activity correlates with the speed and extent of motor execution.37 Abnormal sensorimotor functions in pediatric patients with CSCI may be linked to changes in the corticostriatal sensorimotor circuit, and our results may suggest that MI can improve sensory and motor functions by activating the corticostriatal sensorimotor circuit.
In addition to the reorganization of the corticostriatal circuit, MI of pediatric patients with CSCI also showed higher-level activation in cerebellar lobules III–V, which are located in the anterior cerebellum and play a key role in sensory and motor functions.38 Previous scholars have revealed that increased activation in the anterior cerebellum is related to the spatial accuracy demands of imagining the pointing movement.39 In addition, the cerebellum is known to store internal forward models that can predict movement outcomes, thereby providing precise timing information for predictions.40 The higher-level activation of cerebellar lobules III–V in our results may suggest that MI in pediatric patients with CSCI involves more spatial and temporal accuracy demands after the loss of sensorimotor function.
Limitations.
There are 2 major limitations in our study. First, pediatric patients with CSCI in the current study had varying disease durations. Second, this study was a cross-sectional study, and a future longitudinal study is required to confirm the effect of MI on pediatric patients with CSCI.
CONCLUSIONS
Our study demonstrated that several motor- and cognitive-related areas in the MI network can be functionally preserved in pediatric patients after CSCI. In addition, higher-level activation in sensorimotor-related regions, which reflects functional reorganization, was also detected in pediatric patients with CSCI. The functional preservation and reorganization in the MI network may provide a theoretic basis for the application of MI training in pediatric CSCI.
Acknowledgments
The authors thank the patients and healthy volunteers who participated in this study.
Footnotes
Ling Wang and Weimin Zheng contributed equally to this work and share first authorship.
This study was supported by the National Natural Science Foundation of China (Nos. 81871339 and 81271556), the Beijing Municipal Natural Science Foundation (No:7113155), and the Science Foundation of Beijing Municipal Commission of Education (No. KM201210025013).
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received November 26, 2022.
- Accepted after revision March 16, 2023.
- © 2023 by American Journal of Neuroradiology