Abstract
BACKGROUND AND PURPOSE: While classic brain MR imaging features of Alexander disease have been well-documented, lesional patterns can overlap with other leukodystrophies, especially in the early stages of the disease or in milder phenotypes. We aimed to assess the utility of a new neuroimaging sign to help increase the diagnostic specificity of Alexander disease.
MATERIALS AND METHODS: A peculiar bilateral symmetric hyperintense signal on T2-weighted images affecting the medulla oblongata was identified in an index patient with type I Alexander disease. Subsequently, 5 observers performed a systematic MR imaging review for this pattern by examining 55 subjects with Alexander disease and 74 subjects with other leukodystrophies. Interobserver agreement was assessed by the κ index. Sensitivity, specificity, and receiver operating characteristic curves were determined.
RESULTS: The identified pattern was present in 87% of subjects with Alexander disease and 14% of those without Alexander disease leukodystrophy (P < .001), 3 with vanishing white matter, 4 with adult polyglucosan body disease, and 3 others. It was found equally in both type I and type II Alexander disease (28/32, 88% versus 18/21, 86%; P = .851) and in subjects with unusual disease features (2/2). Sensitivity (87.3%; 95% CI, 76.0%–93.7%), specificity (86.5%; 95% CI, 76.9%–92.5%), and interobserver agreement (κ index = 0.82) were high.
CONCLUSIONS: The identified pattern in the medulla oblongata, called the chipmunk sign due to its resemblance to the face of this rodent, is extremely common in subjects with Alexander disease and represents a diagnostic tool that can aid in early diagnosis, especially in subjects with otherwise atypical MR imaging findings and/or clinical features.
ABBREVIATIONS:
- APBD
- adult polyglucosan body disease
- ADLD
- adult-onset autosomal dominant leukodystrophy
- AxD
- Alexander disease
- GFAP
- glial fibrillary acidic protein
- ION
- inferior olivary nucleus
- IQR
- interquartile range
- VWM
- vanishing white matter
Alexander disease (AxD) is a devastating leukodystrophy caused by gain of function de novo or, less commonly, dominant missense pathogenic variants in the glial fibrillary acidic protein (GFAP) gene.1 Brain MR imaging pattern recognition is crucial in guiding molecular tests to confirm the diagnosis in AxD. Before the identification of the causative genetic etiology of AxD, Van der Knaap et al2 described 5 MR imaging–based criteria for the diagnosis. Several disease classifications are currently in use. The traditional one classifies AxD on the basis of age at disease onset into neonatal (<30 days), infantile (0–2 years), juvenile (2–12 years), and adult (older than 12 years) forms.3,4 AxD can also be classified on the basis of clinical features at onset and brain MR imaging findings in type I and type II AxD.5 Type I generally presents at an earlier age with classic imaging features, including extensive supratentorial leukodystrophy with frontal predominance, brainstem and basal ganglia involvement, and parenchymal enhancement. Type II manifests across the life span, showing atypical imaging features with predominant involvement of the brainstem, and, in some cases, additional supratentorial WM involvement.5 In particular, mild-to-severe atrophy of the medulla oblongata is a common finding with or without signal abnormalities,6 with involvement of the middle cerebellar peduncles.7 Additionally, atypical neuroradiologic features have been identified in AxD, including isolated brainstem involvement and focal MR imaging lesions.8,9
Yoshida et al10 have described bilateral abnormal MR signal of the anterior portion of medulla oblongata in 4 subjects with elderly-onset AxD, with the signal abnormality in the pyramids resembling the “eye spot” of the Taenaris butterfly. We identified a characteristic MR imaging pattern on axial T2-weighted images affecting the medulla oblongata, with bilateral symmetric hyperintensity involving the central inferior olivary nucleus (ION), the pyramids, and the cuneate and gracile fascicles with peripheral ION and inferior peduncle sparing in an index AxD individual and called it the chipmunk sign due to its resemblance to the appearance of the face of this rodent. This study aims to describe this neuroradiologic sign in subjects with AxD and assess its sensitivity and specificity in the diagnostic process of AxD compared with other leukodystrophies and genetic leukoencephalopathies.
MATERIALS AND METHODS
Setting and Participants
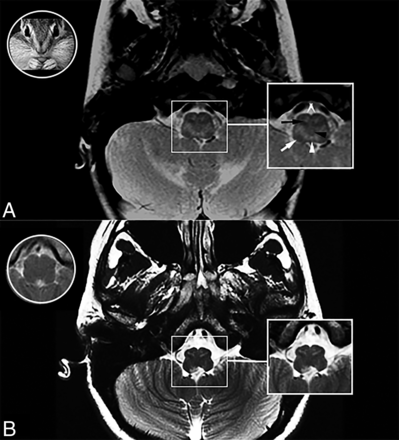
A new MR imaging pattern affecting the medulla oblongata on axial T2-weighted images at the mid-olivary level, resembling a chipmunk face, was identified in an index subject with type I AxD, and it was defined as the chipmunk sign (Fig 1A).
Chipmunk MR imaging sign. T2-weighted axial MRI through the medulla oblongata in subjects with AxD type I (A) and AxD type II (B), an image of a chipmunk face (A, white circle), and a T2WI axial scan of the medulla oblongata in a healthy subject (B, white circle). A typical chipmunk sign in AxD type I (white box in A) and AxD type II (white box in B). The black arrow indicates the inferior olivary nucleus; black arrowhead, medial lemniscus; white arrow, inferior peduncle; white arrowhead, medial longitudinal fasciculus; double white arrows, pyramidal tracts.
Fifty-five additional subjects with pathogenic variants in GFAP for whom at least axial T2-weighted images from brain MR imaging were available in electronic digital format were enrolled in the study. Seventy-four subjects with other leukodystrophies and genetic leukoencephalopathies confirmed by molecular testing, with potential brainstem involvement on MR imaging, were included for comparison (see Online Supplemental Data for details).11 All participants were recruited from 5 different centers (Children’s National Health System, Washington DC, center 1; Children’s Hospital of Philadelphia, Philadelphia, center 2; Fondazione IRCCS Istituto Neurologico “Carlo Besta,” Milan, Italy, center 3; Hospital for Sick Children Toronto, Ontario, Canada, center 4; Montreal Children’s Hospital, Québec, Canada, center 5). Institutional review board approval was obtained at each respective institution. Informed consent for the use of deidentified data for scientific purposes was obtained from all the subjects who participated in clinical investigations.
MR Imaging Evaluation
A single axial T2-weighted image in TIFF for each MR imaging was obtained at the mid-olivary level of the medulla oblongata from the 55 subjects with AxD and 74 with non-AxD leukodystrophy. All images were assigned to a randomized order number (STATA randomization function; StataCorp) and included without additional clinical and/or MR imaging data in a blinded PowerPoint file (Microsoft). All the slides were scored for the presence or absence of the chipmunk sign (T2-hyperintense signal changes of the dorsal medulla, pyramids, and central olives and T2-hypointense signal changes of the peripheral ION and inferior peduncles) by 5 independent examiners (3 pediatric neurologists with expertise in leukodystrophies (A. Vanderver, T.A., D.T. with >10 years’ experience in leukodystrophies) and 2 pediatric neuroradiologists (L.F., M.T.W. with >12 years’ experience). The images were also scored independently for the presence of T2-hyperintense signal changes of the medial lemniscus and T2-hypointense signal of the medial longitudinal fasciculus. The presence of atrophy and/or lesional mass effect was evaluated as well. The chipmunk sign and the other MR imaging features were considered positive by consensus if ≥3 observers considered it positive. When available, an additional file with a single axial T2-FLAIR image at the same level was assessed by the same procedure and reviewed separately from the T2-weighted images to assess the sensitivity and specificity of the chipmunk sign on T2-FLAIR sequences as well.
Statistical Analysis
Interobserver agreement was assessed by the κ index for multiple observers. Comparative analyses on demographics were performed using the Fisher exact test and the χ2 test, Student t test, or Wilcoxon rank-sum test when appropriate. The confidence interval (95%) of the sensitivity and specificity of the chipmunk sign in the diagnosis of AxD was assessed by the Wilson procedure. Multivariate binary logistic regression was used to evaluate age at the time of MR imaging and type of AxD as possible predictors of the presence of the chipmunk sign in subjects with AxD.
RESULTS
Fifty-five subjects with AxD were included in this study (32 type I MR imaging patterns, 21 type II MR imaging patterns, and 2 with an atypical MR imaging pattern with predominant supratentorial, bilateral, and symmetric occipital involvement). Among the 32 subjects with the type I AxD MR imaging pattern, 23 developed a clinical picture classical for type I AxD (early infantile onset, macrocephaly, delayed motor skills, and seizures), but 8 had atypical clinical features, such as substantial brainstem–related symptoms and slow progression, clinically resembling type II AxD.
The median age at the time of brain MR imaging was 2.1 years (interquartile range [IQR], 1.6–5.5 years) for AxD type I MR imaging pattern; 39 years of age (IQR, 16.4–54.0 years) for AxD type II MR imaging pattern; 2.0 years of age (IQR, 0.8–3.1 years) for subjects with an atypical MR imaging pattern; and 2.9 years of age (IQR, 0.7–9.3 years; range, 0–81 years) for subjects with other leukodystrophies and genetic leukoencephalopathies.
Interobserver agreement in assessing the presence or absence of the chipmunk sign in subjects with AxD and a comparison group among the 5 reviewers was excellent (κ index = 0.82).
Medullary signal abnormalities resembling the chipmunk sign were found more frequently in subjects with AxD (48/55, 87%) than in the comparison non-AxD leukodystrophy group (10/74, 14%; OR = 43.8; 95% CI, 15.5–123.6; P < .001). This sign was found equally in both type I and type II AxD (28/32, 88% versus 18/21, 86%; OR = 1.17, P = .851) (Figure and Online Supplemental Data), including the subjects with type I MR imaging but atypical clinical features (n = 8) and in the subjects with an atypical MR imaging pattern with predominant supratentorial, bilateral, occipital involvement (n = 2). Among the subjects with AxD, a multivariate logistic analysis showed that neither AxD type nor age at the time of MR imaging was a predictor of the presence of the chipmunk sign (P = .865 and P = .303).
Subjects with AxD with a type II MR imaging pattern showed more frequent T2-hyperintensity in the medial lemniscus and atrophy but less frequent T2-hypointense signal in medial longitudinal fasciculus than subjects with AxD with a type I MR imaging pattern (P < .05 in all) (Table). No other differences in the involvement of anatomic structures of the medulla oblongata were found when comparing both subgroups of subjects with AxD. In some subjects with AxD, the chipmunk sign was considered atypical due to the coexistence with additional signal abnormalities (Table).
| AxD | Non-AxD | AxD Type I | AxD Type II | VWM | APBD | Other | |
|---|---|---|---|---|---|---|---|
| Total MRIs reviewed | 55 | 74 | 32 | 21 | 6 | 4 | 67 |
| Positive chipmunk sign | 48 | 10 | 28 | 18 | 3 | 4 | 3 |
| T2-hyperintense dorsal medulla | 52 | 17 | 29 | 21 | 5 | 4 | 8 |
| T2-hyperintense pyramids | |||||||
| No | 8 | 59 | 7 | 1 | 2 | 0 | 57 |
| Yes, asymmetric | 5 | 1 | 2 | 3 | 0 | 0 | 1 |
| Yes, symmetric | 42 | 13 | 23 | 17 | 4 | 4 | 5 |
| T2-hyperintense central ION | |||||||
| No | 8 | 58 | 6 | 2 | 1 | 0 | 57 |
| Yes, asymmetric | 5 | 1 | 1 | 4 | 0 | 0 | 1 |
| Yes, symmetric | 41 | 13 | 24 | 15 | 4 | 4 | 5 |
| T2-hypointense peripheral ION | |||||||
| No | 6 | 62 | 4 | 2 | 2 | 0 | 60 |
| Yes, asymmetric | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| Yes, symmetric | 47 | 11 | 27 | 18 | 4 | 4 | 3 |
| T2-hypointense IP | |||||||
| No | 7 | 58 | 4 | 3 | 1 | 0 | 57 |
| Yes, asymmetric | 3 | 1 | 1 | 2 | 0 | 0 | 1 |
| Yes, symmetric | 45 | 14 | 27 | 16 | 5 | 3 | 6 |
| T2-hyperintense ML | 38 | 12 | 19 | 18 | 4 | 4 | 4 |
| Atrophy | 20 | 7 | 4 | 16 | 1 | 4 | 2 |
| Mass effect lesions | 5 | 2 | 3 | 2 | 0 | 0 | 2 |
| T2-hypointense MLF | 35 | 8 | 25 | 8 | 3 | 3 | 2 |
Note:—MLF indicates medial longitudinal fasciculus; ML, medial lemniscus; IP, inferior peduncle.
↵a See the Online Supplemental Data for details on patients and the comparison group.
Involvement of different anatomic structures of the medulla oblongata and additional MR imaging features in subjects with AxD and non-AxD leukodystrophy on T2-weighted imagesa
The small subgroup of subjects with other leukodystrophies and an MR imaging pattern resembling the chipmunk sign were the following: 4/4 with adult polyglucosan body disease (APBD), 3/6 with vanishing white matter (VWM), 3 others (1 with 4H leukodystrophy, 1 with adult-onset autosomal dominant leukodystrophy [ADLD]), and 1 with Pelizaeus-Merzbacher disease. However, these leukodystrophies are easily distinguishable from AxD on the basis of concurrent differing supratentorial brain MR imaging patterns (Online Supplemental Data). Furthermore, the reduced diffusivity of WM structures that is common in VWM and some mitochondrial disorders is not an expected imaging finding in AxD.
Overall, the sensitivity and specificity of the chipmunk sign in the diagnosis of AxD compared with other leukodystrophies were 87.3% (95% CI, 76.0%–93.7%) and 86.5% (95% CI, 76.9%–92.5%), respectively, when only brainstem imaging was reviewed, and the area under the receiver operating characteristic curve was 0.86 (95% CI, 0.80–0.92). Among the 28 subjects (14 with AxD, 14 with non-AxD leukodystrophies) in whom axial T2-FLAIR images were available, detection of a chipmunk sign in this sequence showed lower interobserver agreement (κ index = 0.63) and lower sensitivity (57.1%; 95% CI, 32.6%–78.6%), specificity (78.6%; 95% CI, 52.4%–92.4%), and area under the curve (0.687; 95% CI, 0.51–0.87) compared with T2-weighted images. In addition, in 7/11 (64%) subjects in whom this sign was considered positive on just axial T2-FLAIR images, it was categorized as mild or partial.
DISCUSSION
AxD is known to have characteristic MR imaging features.2,8,9 Brain involvement in the typical form is characterized by a frontal predominance, periventricular T1 and T2 shortening, deep gray nuclear involvement, brainstem involvement, and contrast enhancement. Before the availability of molecular genetic testing of GFAP, when brain biopsy was the mainstay of diagnosis, if 4 of 5 of these criteria were satisfied, neuroimaging was considered diagnostic. However, the neuroimaging phenotype may, in some cases, be variable, and diagnostic criteria may be incompletely fulfilled early in the disease process, especially in mild phenotypes and in atypical variants, particularly in type II AxD.1⇓⇓–4 Additional features are helpful to establish the diagnosis in these subjects.
We have identified an additional imaging pattern that can be seen in subjects with AxD. Specifically, hyperintense signal on T2-weighted images infiltrating the medullary pyramids, central ION, and the dorsal medulla, often involving the regions of the cuneate and gracile fascicles and sparing the inferior cerebellar peduncles. The medial longitudinal fasciculus is often spared as well. Signal alteration affecting the medulla oblongata resembles the face of a chipmunk. The residual T2 hypointensity of the ION corresponds to the chipmunk’s eyes, while spared inferior cerebellar peduncles form the cheeks.
When the chipmunk sign is seen, the remainder of the MR imaging should be analyzed for features consistent with AxD, even in the presence of atypical brain MR imaging features such as occipital involvement or isolated brainstem involvement in adults. In particular, tumorlike brainstem lesions can be found in patients with AxD.12 In these cases, correct and prompt diagnosis can be challenging because lesion biopsy would be expected to reveal Rosenthal fibers, which are common in various neoplastic CNS conditions, including pilocytic astrocytoma.13 The coexistence of the chipmunk sign could obviate the need for biopsy in these circumstances.
The only genetic disorders other than AxD that consistently exhibited a similar pattern of medullary signal in our study were APBD and VWM. However, AxD can be easily distinguished from these disorders from both a clinical and neuroimaging perspective. Unlike AxD, VWM manifests restricted diffusion in the involved WM early on and ultimately causes rarefaction of WM signal that approaches the CSF signal. Clinically, it is often characterized by an evident sensitivity to febrile infections and minor head trauma, which may cause a rapid neurologic deterioration with often marked cerebellar ataxia.14,15 In APBD, the cerebral WM involvement is more diffuse, lacking evidence of a disease gradient, and subjects usually develop neurogenic bladder, progressive spastic gait, and peripheral neuropathy.16 Further studies may be useful, however, to further characterize these medullary imaging findings in APBD.
Limitations of this study include the retrospective design and the possibility of observer bias. Against this confound, the sensitivity was only 87.3% on T2-weighted images and 57.1% on T2-FLAIR images. Moreover, due to the rarity of the disease, the sample size is relatively small, which may affect the external validity of the chipmunk sign, though the high prevalence of this sign further supports our analyses. Further prospective blinded studies may be useful to validate our findings.
CONCLUSIONS
We have suggested an imaging finding called the chipmunk sign to be characteristic of AxD in the appropriate clinical and MR imaging context, after excluding a few other metabolic and genetic conditions. Our approach may help physicians consider this disorder earlier in the diagnostic odyssey, particularly in subjects with otherwise atypical MR imaging findings and/or clinical features. Subjects with VWM or APBD can show a similar brainstem involvement, but clinical features as well as additional supratentorial MR imaging features easily help to distinguish them from AxD. Identification of the chipmunk sign should prompt careful assessment for other supportive AxD imaging findings and consideration of genetic studies for the sequencing of GFAP.
Footnotes
↵ T. Armangue and M.T. Whitehead contributed equally to the article.
This study was partially supported by Children’s National Medical Center, Department of Neurology (A. Vanderver), Carlos III Spanish Health Institute (CM14/00081, CV15/00021 TA).
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received November 29, 2023.
- Accepted after revision February 1, 2024.
- © 2024 by American Journal of Neuroradiology