SUMMARY:
Epstein-Barr virus, a herpesvirus, has been associated with a variety of cancers, including Burkitt, Hodgkin, and non-Hodgkin lymphomas; posttransplant lymphoproliferative disorders; gastric carcinoma; and nasopharyngeal carcinoma, in both immunocompetent and immunocompromised individuals. Previous studies have established a connection between Epstein-Barr virus and the development of smooth-muscle tumors. Smooth-muscle tumors of the brain are very rare and are often misdiagnosed as meningiomas on imaging. To our knowledge, advanced imaging findings such as MR perfusion of smooth-muscle tumors of the brain have never been reported. We describe the radiologic and pathologic features of the Epstein-Barr virus–associated smooth-muscle tumors of the brain in a person with newly diagnosed advanced HIV.
ABBREVIATIONS:
- EBV
- Epstein-Barr virus
- EBV-SMT
- Epstein-Barr virus–associated smooth-muscle tumor
- rCBV
- relative CBV
- SMT
- smooth-muscle tumor
A 35-year-old African-Caribbean man presented with a history of gait instability, confusion, and sensorial changes. Four weeks before the onset of the neurologic symptoms, he had presented to our hospital with a 3-week history of fevers, chills, sweats, and weight loss. He was diagnosed at that time with advanced HIV, with an initial CD4 count of 0 and HIV viral load of 410,000 copies/mL. He was started on antiretroviral therapy with bictegravir/emtricitabine/tenofovir alafenamide (Biktarvy). When presenting with his new neurologic changes, he was found to be confused but able to obey commands with prodding, with bilateral dysmetria, worse on the right side. There were no other focal neurologic deficits.
Imaging
MR imaging demonstrated a solitary, rounded mass lesion located in the posterior medial aspect of the right posterior fossa. It had a broad dural base abutting the inferior surface of the right tentorium cerebelli without dural thickening or a dural tail sign. The lesion measured up to 2.8 cm in maximum diameter. On pregadolinium T1WI, the lesion was predominantly isointense to the cerebellar cortex (Fig 1A). On T2WI and FLAIR, the lesion was predominantly hypointense to the cerebellar cortex (Fig 1B, -C). On postgadolinium T1WI, the lesion demonstrated solid, intense enhancement with minimal foci of necrosis peripherally (Fig 1F, -G). There was associated perilesional edema causing mild effacement of the fourth ventricle (Fig 1B, -C). There was no diffusion restriction on DWI (Fig 1D). A few small blooming foci were noted within the lesion seen on SWI (Fig 1E). On DSC MR perfusion-weighted imaging, the mass had low relative cerebral blood volume (rCBV) (Fig 1H).
Tumor appearance on MR imaging. MR images show a right posterior fossa mass, which had isointense signal on pregadolinium T1WI (A) and hypointense signal on T2WI (B) and FLAIR (C) with perilesional edema (B and C). The mass does not show diffusion restriction on DWI (D). There are a few blooming foci within the tumor seen on SWI (E). The mass shows intense enhancement with multifocal, small, nonenhancing areas of necrosis on axial postgadolinium T1WI (F). Sagittal postgadolinium T1WI (G) demonstrates the tumor attached to the inferior surface of the right tentorium cerebelli (arrow). On DSC MR perfusion (H), the tumor had low rCBV.
Subsequent Clinical Course and Operative Report
Given the patient’s history of HIV with a low CD4 count and high viral load, the clinical team was very concerned that the intracranial lesion might represent an atypical opportunistic infection, particularly CNS toxoplasmosis, or an HIV-related neoplasm, with CNS lymphoma being a specific concern, despite the imaging appearances not being typical for either condition. Consequently, the clinical team decided to start empiric treatment for CNS toxoplasmosis with pyrimethamine, leucovorin, and clindamycin, in the context of prior adverse drug reaction to trimethoprim-sulfamethoxazole (angioedema and rash). He also had extensive abdominal lymphadenopathy, prompting concern for atypical infection or HIV-related lymphoma with metastatic spread, and a lymph node biopsy was performed, diagnosing disseminated Mycobacterium avium complex. He was started on an appropriate therapy. Due to the lack of response of the intracranial lesion as determined on repeat imaging with empiric treatment at 2 weeks, surgical resection was performed. The goals of surgery were the following: a definitive tissue diagnosis, control of mass effect leading to improvement in symptoms, and possible oncologic control with a safe, maximal resection. He underwent suboccipital craniectomy and gross total resection of the tumor. Intraoperatively, we found that the lesion was extra-axial, intradural, and well-demarcated from the surrounding brain with a dural attachment to the tentorium. The tumor was tan-gray and firm and rubbery in consistency. He tolerated the surgery well and was discharged with his condition improved and complete resolution of his neurologic deficits. Subsequent MR imaging at the 1-year follow-up revealed no evidence of tumor recurrence.
Pathology
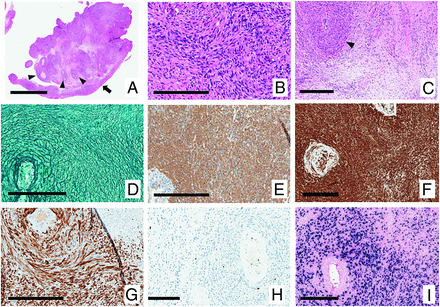
Histologic examination revealed a tumor made up of cells with spindle-shaped nuclei with blunted ends and wavy eosinophilic cytoplasm of uncertain limits (Fig 2B). Tumor cells were arranged in intersecting streams and whorls surrounding blood vessels, which often had thick, partially hyalinized walls (Fig 2A, -C). There were no tight whorls. The tumor attachment to the dura mater was demonstrated (Fig 2A). Areas of necrosis were scattered throughout, while mitotic activity was inconspicuous. Reticulin stains demonstrated wrapping of single cells, with no reticulin-free areas (Fig 2D). Immunohistochemistry showed positive expression of smooth-muscle actin (Fig 2E) and myosin (Fig 2F), with only a subset of cells expressing desmin (Fig 2G). Scattered CD3-positive T lymphocytes were scattered throughout, with no CD20-positive B lymphocytes. The Ki-67 proliferation index was approximately 2%, high only in the lining of blood vessels (Fig 2H). Epstein-Barr virus (EBV)-encoded RNA in situ hybridization was intensely positive (Fig 2I). The tumor cells were negative for EMA, SOX-10, STAT6, and E-cadherin. Given these features, the definitive diagnosis was an EBV-associated smooth-muscle tumor (EBV-SMT). Meningioma was ruled out by the absence of tight whorls and expression of EMA and E-cadherin, as well as reticulin-free areas. Solitary fibrous tumor was excluded by the negative for STAT6.
Histopathology. H&E-stained sections A, B, and C (magnification A, bar 4 mm; B, bar 200 um; and C, bar 300 um). A, The attachment to the dura mater is demonstrated at the lower right (arrow). B, Cells have spindle-shaped nuclei with blunted ends and wavy eosinophilic cytoplasm of uncertain limits and are arranged in intersecting streams and whorls. The tendency to surround blood vessels is seen in A and C (arrowheads). Reticulin stains demonstrate wrapping of single cells, with no reticulin-free areas (D, bar 200 um). Immunohistochemistry shows expression of smooth-muscle actin (E, bar 200 um) and myosin (F, bar 200 um) with only a subset of cells expressing desmin (G, bar 200 um). The Ki-67 proliferation index was approximately 2%; high only in the lining of blood vessels (H, bar 200 um). EBV-encoded RNA in situ hybridization was intensely positive (I, bar 200 um).
DISCUSSION
EBV, a herpesvirus, has been linked to several types of tumors, including Hodgkin and non-Hodgkin lymphoma and nasopharyngeal carcinoma, in both immunocompetent and immunocompromised patients.1⇓⇓⇓-5 Several reports have found a link between EBV and the development of smooth-muscle tumors (SMTs), particularly in immunocompromised patients such as transplant recipients and people living with HIV, particularly those with low CD4 counts.2,5⇓-7 It is believed that immune suppression predisposes patients to the development of SMTs.1,2,4,5,8⇓-10
EBV-SMTs have been variably labeled, ranging from “leiomyoma” to “smooth-muscle tumor of uncertain malignant potential” to “leiomyosarcoma.”2,11,12 Approximately one-half of patients with EBV-SMT had multifocal lesions because the tumor can occur as multiple synchronous masses.2,3,5 Unlike conventional somatic SMTs, which tend to follow the distribution of smooth muscle throughout the body, EBV-SMTs exhibit an unusual predilection for atypical locations where there is little smooth muscle. The most common site of EBV-SMTs is the intracranial dura mater.2,4,5,7 Extracranial involvement has also been reported in various organs, including the spine, adrenal gland, lung, spleen, gallbladder, bone, bladder, nasopharynx, oropharynx, larynx, thyroid, and heart.2,4,5
Although the exact origin of intracranial SMTs remains uncertain, EBV-associated SMTs exhibit distinct characteristics compared with conventional somatic SMTs. Intracranial EBV-SMTs appear closely associated with the walls of small blood vessels.2,5 The progenitor cell for EBV-SMT is believed to originate from an aberrant myogenous vascular smooth-muscle cell. It has been demonstrated that normal smooth-muscle cells express the CD21 receptor. In the case of EBV-SMT, EBV directly infects the smooth-muscle cells by binding to CD21, thereby facilitating and promoting replication within this cell. Another proposed pathogenic mechanism is the fusion of EBV-infected lymphocytes with smooth-muscle cells, which may play a critical role in disease development. Nevertheless, the mechanism of EBV-SMT clonal proliferation following EBV infection is still unknown.1⇓⇓⇓-5
Radiologically, EBV-SMTs are typically well-circumscribed extra-axial masses. These tumors typically demonstrate hypointense signal on T2-weighted imaging due to the T2-shortening effect of intramuscular actin, myosin, and collagen and densely packed cellular architecture relative to the surrounding brain tissue.13,14 A few reports described the tumors as having a central hyperintensity core due to central necrosis.6,7,15 They also tend to appear iso- to hypointense on T1-weighted imaging and usually show intense enhancement on postcontrast study. The frequency of necrosis or hemorrhage varies among case reports.6,7 The presence of peritumoral edema is also variable and is probably related to the aggressiveness of the tumor or associated infection. These tumors tend to be devoid of calcifications and do not demonstrate diffusion restriction.7 Although these tumors are primarily extra-axial in origin, they do not exhibit bone involvement or remodeling.2⇓-4,16⇓-18 To date, MR perfusion findings in EBV-SMT of the brain have never been reported.
The diagnosis of EBV-SMT of the brain on imaging can be challenging, because radiologic features often overlap with more common extra-axial brain tumors, particularly meningiomas.2,5,18,19 In our case, the tumor was located in the periphery of the posterior fossa, making the differentiation between intra- and extra-axial locations radiologically challenging. The superficial location and broad dural-based abutment of the adjacent tentorial dura suggested an extra-axial origin of the lesion. The presence of low rCBV on DSC MR perfusion imaging allowed us to exclude more common posterior fossa tumors like meningiomas, hemangioblastoma, and hemangiopericytomas, which typically exhibit higher rCBV values. Additionally, the absence of diffusion restriction made CNS lymphoma, a common HIV-associated CNS neoplasm, less likely. Given the history of HIV infection with a low CD4 count, EBV-SMT of the brain was raised as a diagnostic possibility. There are several atypical opportunistic CNS infections that can present with intracranial masslike lesions, such as toxoplasmosis, cryptococcoma, tuberculoma, cytomegalovirus (CMV), and syphilitic gumma. An extra-axial location, lack of diffusion restriction, and no response to empiric treatment based on MR imaging findings made toxoplasmosis and other atypical opportunistic infections less likely. The negative results from microbiologic or serologic testing were reassuring.
DSC MR perfusion is an advanced MR imaging technique that can provide more information on the vascularity and angiogenesis degree of the tumor.20,21 MR perfusion measurements of rCBV have been shown to correlate with both conventional angiographic assessments of tumor vascular density and histologic measures of tumor neovascularization. However, increased tumor vascularity does not necessarily imply malignancy, especially for extra-axial tumors, such as meningiomas, hemangiopericytomas, and paragangliomas, which generally demonstrate high rCBV values.20 In our patient with EBV-SMT, even though pathologically this tumor has a predilection for the smooth muscles of the blood vessel walls, the tumor was not hypervascular and did not have marked neoangiogenesis based on our MR perfusion findings.
While MR perfusion is not routinely performed for assessing extra-axial intracranial masses, it has proved helpful in distinguishing less common extra-axial tumorlike lesions such as Rosai-Dorfman disease, Erdheim-Chester disease, and neurosarcoidosis, which typically display low rCBV values, from more common tumors like meningiomas, which are hypervascular and exhibit high rCBV values on conventional MR imaging.19,22⇓⇓-25 Adding MR perfusion could aid in distinguishing EBV-SMTs from meningiomas in patients with HIV presenting with atypical enhancing extra-axial masses.
There is limited literature available on nuclear scintigraphy and molecular imaging findings in EBV-SMTs. However, a few case reports have shown that [18F] FDG-PET/CT could be helpful in detecting multifocal synchronous EBV-SMTs in different organs and for assessing the treatment response.26,27 Recent studies have demonstrated that PET/CT imaging using radiolabeled somatostatin receptor ligands such as gallium 68 [68Ga] DOTATOC or [68Ga]-DOTATATE PET/CT may assist in distinguishing meningiomas from other extra-axial masses, including dural-based metastases, paragangliomas, schwannomas, and nontumor lesions.28,29 However, studies focusing on somatostatin receptor PET for differentiating meningiomas and EBV-SMTs have not been reported to date.
To our knowledge, there is no standardized therapy for intracranial or spinal EBV-SMTs. Surgical resection continues to be the primary treatment. In instances in which this is deemed feasible, a safe, maximal resection provides a definitive diagnosis, allows control of mass effect, and intuitively leads to improved oncologic control. Combining surgery with adjuvant radiation therapy or chemotherapy is an emerging therapeutic option that should be considered. Immune reconstitution is advised for all patients, given that the development of EBV-SMTs is strongly linked to an immunocompromised state.3⇓-5,30
Case Summary
EBV-SMTs constitute a very rare oncologic entity. They usually develop in the context of secondary immunodeficiency caused by HIV infection or immunosuppressive treatment after solid organ transplantation.
EBV-SMTs can manifest as solitary or multiple lesions. The most common site of these tumors is the intracranial dura mater, which usually mimics meningiomas on imaging.
EBV-SMTs typically show hypointense signal relative to the surrounding brain on T2WI due to the presence of smooth-muscle cells.
MR perfusion has been demonstrated to be helpful in distinguishing meningiomas from their mimics. In our patient with EBV-SMT, the tumor demonstrated low rCBV values on MR perfusion. This characteristic may be helpful in the differentiation from meningiomas, which typically present as hypervascular tumors and with elevated CBV values.
The primary treatment recommendation for EBV-SMTs is surgical resection, with or without combining adjunctive therapies, along with treatment of the underlying immunodeficiency.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received December 4, 2023.
- Accepted after revision February 14, 2024.
- © 2024 by American Journal of Neuroradiology